Introduction to Reference Electrodes
Definition and Role
Reference electrodes (RE) serve as indispensable components in electrochemical systems, primarily functioning to provide a stable and known potential. This stability is crucial for accurately analyzing the potential of working electrodes, thereby enabling precise measurements of electrode states. By offering a consistent reference point, REs effectively decouple the electrode states, facilitating a clearer understanding of the electrochemical processes occurring within the system.
In practical applications, such as in lithium-ion batteries, the role of reference electrodes extends beyond mere measurement. They are essential for both qualitative and quantitative characterization, playing a pivotal role in battery testing and failure analysis. The stability and reliability of REs ensure that the data obtained is accurate and reproducible, which is vital for advancing battery technology and improving performance.
Moreover, the choice of reference electrode can significantly impact the outcomes of electrochemical studies. Different types of REs, such as hydrogen standard electrodes, saturated calomel electrodes, and silver-silver chloride electrodes, each have their unique advantages and are selected based on specific requirements of the system. For instance, in environments with varying pH levels, electrodes like the calomel electrode are particularly useful due to their adaptability.
In summary, reference electrodes are not just passive components in electrochemical systems; they are active participants that ensure the integrity and accuracy of experimental data. Their role in providing a stable reference potential is fundamental to the successful analysis and interpretation of electrode states in various electrochemical applications.

Basic Requirements
Reference electrodes are indispensable components in electrochemical systems, particularly in the context of lithium-ion batteries. They serve as the backbone for accurate potential measurements, ensuring the reliability and precision of experimental results. To fulfill this critical role, reference electrodes must meet several stringent requirements.
First and foremost, stability is paramount. The electrode potential must remain constant over extended periods, even under varying experimental conditions. Fluctuations in potential can lead to erroneous data, compromising the integrity of the research. This stability is often achieved through the careful selection of materials and the meticulous design of the electrode structure.
Reversibility is another key attribute. The electrode should be capable of undergoing repeated oxidation and reduction processes without degradation. This ensures that the electrode can be reused multiple times, reducing costs and environmental impact. High reversibility also implies that the electrode can accurately measure potentials during both charging and discharging cycles, providing a comprehensive understanding of the electrochemical system.
Lastly, non-interference is crucial. The reference electrode must not introduce any contaminants or alter the composition of the electrolyte. This is particularly important in lithium-ion batteries, where even trace impurities can significantly affect performance and safety. Ensuring that the electrode remains inert and does not react with the surrounding environment is essential for maintaining the purity and integrity of the electrochemical system.
In summary, the basic requirements for reference electrodes—stability, reversibility, and non-interference—are not just technical specifications but fundamental pillars that underpin the accuracy and reliability of electrochemical studies.
Types of Reference Electrodes
Hydrogen Standard Electrode
The hydrogen standard electrode (SHE) is a cornerstone in electrochemical systems, serving as the universal reference for measuring electrode potentials. This reference electrode is composed of an inert platinum surface on which hydrogen gas is adsorbed, submerged in a solution containing hydrogen ions at unit activity. The SHE's half-cell reaction is represented by the equation:
$$2H^+(aq) + 2e^- \leftrightarrow H_2(g)$$
with an arbitrarily assigned half-cell potential of zero (E0 = 0.000 V). This standardized potential allows for the comparison and tabulation of electrode potentials for various redox couples, providing a consistent baseline across different electrochemical studies.

The SHE is particularly valued for its stability over time and under varying temperature conditions, ensuring reproducible and reliable measurements. Its construction adheres to stringent criteria, including the use of half-cell components that maintain well-defined activity levels and exhibit fixed, reproducible electrode potentials. This makes the SHE an indispensable tool in the calibration and evaluation of other reference electrodes, thereby enhancing the accuracy and comparability of electrochemical data.
Saturated Calomel Electrode
The Saturated Calomel Electrode (SCE) is a widely used reference electrode, particularly advantageous in various pH environments. This electrode is composed of a half-cell consisting of mercurous chloride (Hg₂Cl₂, calomel) in contact with mercury metal, either as a pool or as a paste mixed with calomel. These components are typically layered under a saturated solution of potassium chloride (KCl) or enclosed within a fritted compartment surrounded by the saturated KCl solution, known as a double-junction arrangement. A platinum wire is commonly employed to facilitate contact with the external circuit.
The half-reaction of the SCE is described by the equation:
$$ Hg₂Cl₂(s) + 2e⁻ ⇌ 2Hg(l) + 2Cl⁻(sat′d) $$
This reaction yields a potential of 0.241 V relative to the Standard Hydrogen Electrode (SHE) at 25°C. The double-junction arrangement of the SCE, as illustrated in Figure 34, ensures that contact with the electrochemical cell is made through a porous glass frit or fiber, which permits ion exchange without allowing bulk mixing of electrolytes.
The SCE's construction, involving a solid paste of Hg₂Cl₂ and liquid elemental mercury attached to a rod immersed in a saturated KCl solution, makes it relatively easier to fabricate and maintain compared to other reference electrodes like the SHE. The necessity for a saturated KCl solution is crucial as it fixes the activity by the potassium chloride, resulting in a lower and more stable voltage closer to the SHE. This saturated solution facilitates the exchange of chlorine ions, ensuring the electrode's functionality. Typically, all these components are housed within a tube featuring a porous salt bridge to allow electrons to flow back through and complete the circuit.
Silver-Silver Chloride Electrode
The Silver-Silver Chloride (Ag/AgCl) electrode is a widely preferred choice for various electrochemical applications, particularly when stability and reliability are paramount. This electrode is composed of a silver wire coated with a layer of solid silver chloride (AgCl), which is then immersed in a solution saturated with both KCl and AgCl. The electrode's half-reaction can be represented as:
AgCl(s) + e⁻ ⇌ Ag(s) + Cl⁻(sat′d)
At 25°C, this reaction yields a potential of 0.197 V relative to the Standard Hydrogen Electrode (SHE), which is slightly different from the standard potential (0.222 V) due to the influence of both KCl and AgCl on chloride activity.
Key Features of Ag/AgCl Electrode
- Stability and Reliability: The Ag/AgCl electrode offers a stable half-cell potential that remains consistent over time, making it an excellent reference in diverse conditions.
- Temperature Dependence: While its potential exhibits a slight temperature dependence, changing by approximately 0.5 – 1.0 mV/°C, this variation is minimal and manageable in most applications.
- Safety and Cost-Effectiveness: Unlike the Calomel electrode, which contains mercury, the Ag/AgCl electrode is safer and less toxic, contributing to its widespread use.
Construction and Operation
The construction of an Ag/AgCl electrode involves coating a silver wire with AgCl and placing it in a KCl and AgCl saturated solution. This setup allows for the formation and dissolution of ions as electrons flow in and out of the electrode system, ensuring continuous and reliable operation.
In summary, the Silver-Silver Chloride electrode stands out for its robust performance, safety, and cost-effectiveness, making it a go-to choice in numerous electrochemical studies.
Reference Electrodes in Lithium-Ion Batteries
Importance in Battery Research
Reference electrodes play a pivotal role in the research and development of lithium-ion batteries, serving as indispensable tools for both qualitative and quantitative characterization. These electrodes are crucial for accurately measuring the potential differences between various components within the battery, thereby enabling researchers to pinpoint the root causes of performance degradation and failure.
In the intricate process of lithium-ion battery testing, reference electrodes provide a stable and known potential, which is essential for decoupling the electrode states. This decoupling allows for a more precise analysis of the electrochemical reactions occurring within the battery, facilitating the identification of issues such as capacity fade, internal resistance, and cycle life.
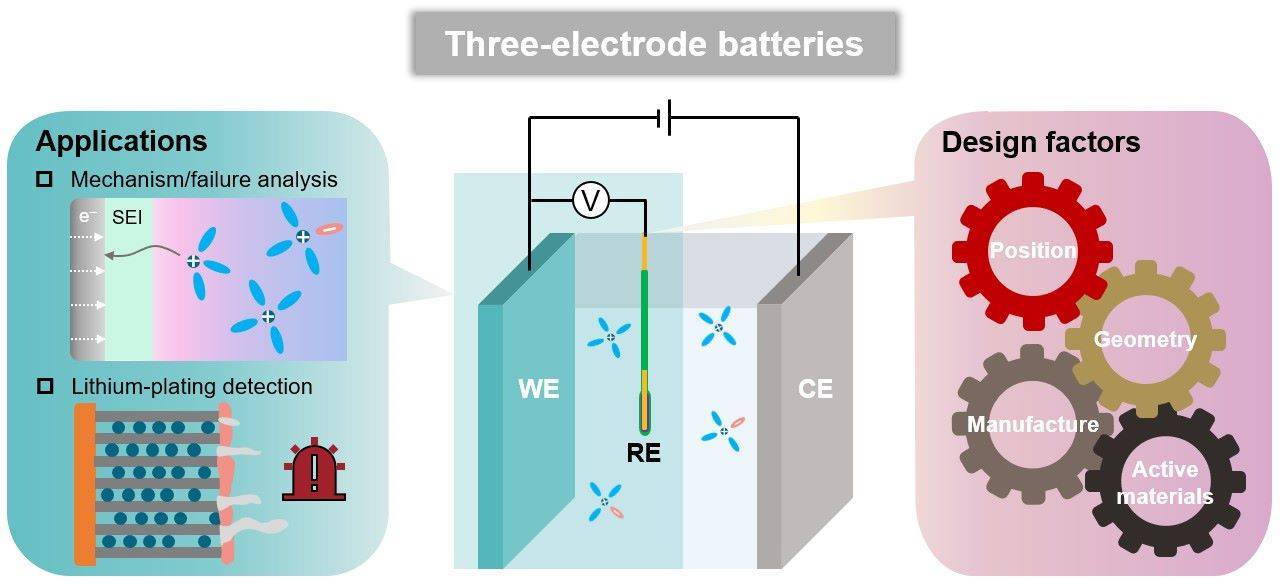
Moreover, reference electrodes are vital in failure analysis, where they help in diagnosing the specific causes of battery malfunctions. By providing a stable reference point, these electrodes allow researchers to isolate and study individual components, such as the anode, cathode, and electrolyte, thereby aiding in the development of more robust and efficient battery designs.
The importance of reference electrodes in lithium-ion battery research extends beyond mere measurement; they are integral to the advancement of battery technology. As the demand for higher energy densities and longer lifespans continues to grow, the role of reference electrodes in ensuring the accuracy and reliability of battery testing and analysis will become even more critical.
Specific Requirements
In lithium-ion batteries, the design and selection of reference electrodes (REs) are critical to ensuring the integrity and efficiency of the battery's operation. These electrodes must be meticulously engineered to be compact and safe, minimizing any potential disruption to the battery's performance. The small size of reference electrodes is essential to avoid spatial interference within the battery, which could lead to inaccurate measurements and compromised battery efficiency.
Safety considerations are paramount, as any hazardous materials or unstable configurations could lead to catastrophic failures, posing significant risks to both the battery and its environment. Therefore, the materials used in these electrodes must be non-toxic and chemically stable under the battery's operational conditions. This ensures that the reference electrode not only provides accurate and reliable potential measurements but also contributes to the overall safety and longevity of the lithium-ion battery system.
| Requirement | Description |
|---|---|
| Size | Must be compact to avoid spatial interference and ensure accurate readings. |
| Safety | Non-toxic and chemically stable to prevent hazardous failures. |
By adhering to these specific requirements, reference electrodes can effectively support the rigorous demands of lithium-ion battery research and applications.
Types of Reference Electrodes in Lithium Batteries
In the realm of lithium-ion batteries, the selection of reference electrodes is crucial for accurate potential measurements and performance evaluations. Several types of reference electrodes are commonly employed, each offering unique advantages and facing distinct challenges.
-
Lithium Metal Electrodes: These are straightforward and widely used due to their simplicity and direct correlation with the lithium ion activity in the electrolyte. However, their use is often limited by the formation of dendrites, which can lead to safety concerns.
-
Lithium Alloy Electrodes: By alloying lithium with other metals, such as aluminum or tin, these electrodes can enhance stability and reduce dendrite formation. This approach, however, introduces complexity in fabrication and may affect the electrode's reversibility.
-
Lithium Oxide Electrodes: These electrodes offer improved stability and reduced reactivity compared to pure lithium electrodes. They are particularly beneficial in high-voltage applications but require careful handling due to their sensitivity to moisture and oxygen.
Each type of reference electrode in lithium-ion batteries presents a trade-off between performance, safety, and ease of use, necessitating careful consideration based on the specific requirements of the battery system.
Challenges and Future Prospects

Current Challenges
Developing long-life and highly stable reference electrodes remains a significant challenge in the field of electrochemistry. The primary obstacles include maintaining consistent electrode potential over extended periods, ensuring high reversibility, and preventing contamination or interference with the electrochemical system. These challenges are particularly acute in applications like lithium-ion batteries, where the reference electrodes must be both small in size and safe, without compromising their stability and reliability.
One of the key issues is the degradation of the electrode material over time, which can lead to fluctuations in potential and reduced accuracy in measurements. This degradation is often exacerbated by environmental factors such as temperature variations and exposure to different electrolyte compositions. Additionally, the need for miniaturization in battery research introduces further complexities, as smaller electrodes must maintain the same level of performance as their larger counterparts.
To address these challenges, researchers are exploring new materials and designs that can enhance the longevity and stability of reference electrodes. For instance, the development of advanced coatings and protective layers aims to shield the electrode from environmental influences while maintaining its electrochemical properties. Furthermore, innovative fabrication techniques are being investigated to create more robust and durable electrode structures.
Despite these efforts, the quest for the perfect reference electrode continues, driven by the need for more accurate and reliable measurements in electrochemical systems. As technology advances, future developments in this field are expected to bring significant improvements, making reference electrodes more versatile and effective in various applications.
Future Developments
Advancements in technology and equipment are poised to revolutionize the performance and applicability of reference electrodes in battery research. These innovations are expected to address key challenges such as the development of long-life and highly stable reference electrodes, which are crucial for the accurate and reliable measurement of electrode potentials.
One promising area of development is the integration of advanced materials, such as nanomaterials and composites, into the construction of reference electrodes. These materials can enhance the electrode's stability and reversibility, making them more suitable for use in diverse and demanding environments, including high-temperature and high-pressure conditions.
Additionally, advancements in microfabrication techniques are likely to lead to the creation of smaller, more efficient reference electrodes. These miniaturized electrodes can be seamlessly integrated into battery systems without compromising the battery's performance or safety. This is particularly important in the context of lithium-ion batteries, where the size and safety of reference electrodes are critical factors.
Moreover, the adoption of smart technologies, such as real-time monitoring and automated calibration, is expected to further improve the accuracy and reliability of reference electrodes. These technologies can help researchers quickly identify and correct any potential issues, ensuring that the reference electrodes remain stable and accurate over extended periods.
In summary, the future of reference electrodes in battery research looks promising, with ongoing technological advancements set to enhance their performance, applicability, and reliability. These developments will not only address current challenges but also open up new possibilities for the advancement of electrochemical systems.
