Introduction to Plant Essential Oils
Definition and Importance
Plant essential oils are a diverse group of aromatic compounds that are typically liquid at room temperature, hence their designation as "essential oils." These oils are renowned for their distinctive scents and multifaceted therapeutic properties, which have garnered widespread appreciation and use in various applications.
The allure of essential oils lies in their unique aromatic profiles, which can range from invigorating citrus notes to soothing floral fragrances, and even to grounding earthy tones. This aromatic diversity is not merely aesthetic; it plays a crucial role in their applications across aromatherapy, perfumery, and culinary arts.
Beyond their olfactory appeal, essential oils are valued for their bioactive properties. These properties can include antimicrobial, anti-inflammatory, and antioxidant effects, among others. This makes them valuable in both traditional medicine practices and modern wellness regimens.
The importance of essential oils extends to their ecological role as well. In plants, these oils often serve as a form of natural defense against pests and pathogens. Understanding and harnessing these natural defenses can lead to more sustainable agricultural practices and the development of eco-friendly pest control methods.
In summary, plant essential oils are not just a collection of aromatic compounds; they are a cornerstone of various industries and practices, driven by their unique scents and therapeutic benefits. Their extraction and utilization are pivotal in both preserving natural resources and enhancing human well-being.

Categories of Essential Oils
Essential oils, renowned for their distinctive aromas and therapeutic properties, are categorized into eight primary groups: Citrus, Floral, Herbaceous, Camphoraceous, Spices, Resinous, Woody, and Earthy. Each category exhibits unique characteristics that contribute to their diverse applications in aromatherapy, culinary arts, and medicinal practices.
-
Citrus Oils: Derived from the peels of citrus fruits such as lemon, orange, and grapefruit, these oils are known for their refreshing and invigorating scents. They are often used to uplift mood and enhance concentration.
-
Floral Oils: Extracted from flowers like rose, jasmine, and lavender, these oils are celebrated for their sweet, soothing aromas. They are frequently employed in skincare products and for their calming effects.
-
Herbaceous Oils: These oils, sourced from herbs like rosemary, thyme, and basil, offer a fresh, green aroma. They are commonly used in culinary preparations and for their invigorating properties.
-
Camphoraceous Oils: Characterized by their cooling and penetrating scents, these oils, including those from eucalyptus and peppermint, are often used in respiratory treatments and to alleviate muscle pain.
-
Spice Oils: Extracted from spices such as clove, cinnamon, and ginger, these oils provide a warm, spicy aroma. They are utilized in both culinary and medicinal contexts, particularly for their antiseptic and anti-inflammatory properties.
-
Resinous Oils: These oils, such as those from frankincense and myrrh, emit a rich, balsamic scent. They are valued for their spiritual and therapeutic uses, often employed in meditation and for their anti-inflammatory effects.
-
Woody Oils: Derived from trees like cedarwood and sandalwood, these oils possess a deep, earthy aroma. They are frequently used in perfumes and for their grounding and calming properties.
-
Earthy Oils: Characterized by their grounding and stabilizing scents, these oils, including patchouli and vetiver, are often used in aromatherapy to promote emotional balance and stability.
Understanding these categories helps in selecting the appropriate essential oils for specific needs, enhancing their efficacy and ensuring a harmonious experience.
Extraction Methods
Water Vapor Distillation
Water vapor distillation is a fundamental method for extracting essential oils from plant materials. The process involves several key steps that facilitate the separation of essential oils from the plant matrix. Initially, water molecules penetrate the cells of the plant material, causing the essential oil components to diffuse into the water. This diffusion leads to the formation of an azeotropic system, where the essential oils and water coexist in a specific ratio.
As the temperature rises, both the essential oils and water vaporize. These vapors then travel through a condenser, where they cool and return to their liquid state. The condensed mixture, now consisting of both water and essential oils, is collected. Due to their different densities, the essential oils float on the surface of the water, allowing for easy separation. This method is particularly effective for extracting oils that are thermally stable and do not degrade under the conditions of steam distillation.
The azeotropic nature of the mixture ensures that the essential oils are not subjected to high temperatures for extended periods, which helps maintain their chemical integrity and aromatic properties. This technique is widely used in the production of essential oils such as lavender, rosemary, and eucalyptus, where the preservation of the oil's natural composition is crucial.
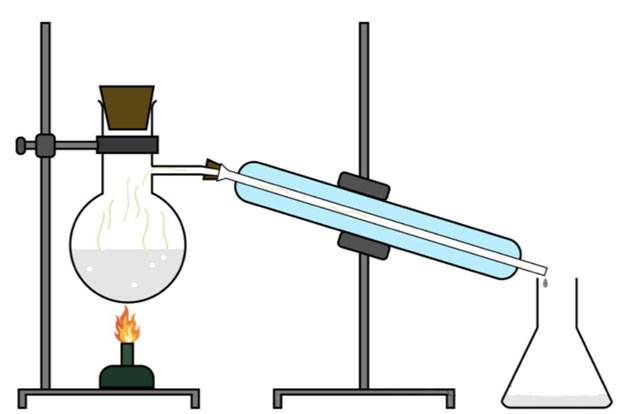
Extraction Method
The extraction method for obtaining essential oils from aromatic plants involves the use of volatile solvents such as petroleum ether, ether, ethanol, benzene, methylene chloride, and gasoline. These solvents are chosen for their ability to effectively leach out the aromatic components from the plant material. The process begins with the immersion of the plant material in the solvent, which facilitates the transfer of essential oil molecules into the solvent phase.
Once the aromatic components have been extracted, the solvent is then evaporated and concentrated to recover the aromatic compounds. This results in a mixture that contains not only the desired essential oils but also various impurities such as waxes, pigments, fats, and other non-volatile materials. To obtain a purer form of essential oil, this mixture undergoes a refining process. This step is crucial as it separates the essential oils from the impurities, ensuring that the final product is of high quality and free from contaminants.
The choice of solvent is significant as it directly influences the efficiency and purity of the extraction process. Different solvents have varying degrees of volatility and selectivity, which can affect the composition and quality of the extracted essential oils. For instance, solvents with lower boiling points, like ether, allow for easier evaporation and concentration, thereby simplifying the recovery process. On the other hand, solvents with higher selectivity, such as ethanol, can target specific aromatic compounds more effectively, leading to a more refined final product.
In summary, the extraction method leverages the properties of volatile solvents to isolate essential oils from plant material. The process involves solvent immersion, evaporation, concentration, and refining to produce a mixture that is rich in essential oils but also laden with impurities. The subsequent refining step is essential to purify the essential oils, ensuring that the final product meets the desired standards of quality and purity.
Adsorption Method
The adsorption method leverages the physical adsorption properties of aromatic plants to capture their volatile components. This technique involves transferring these volatile compounds to an adsorption medium, which can later be desorbed to recover the essential oils.
One of the key advantages of this method is that it preserves the chemical integrity of the original aroma. The essential oils extracted through adsorption remain unchanged, ensuring that their unique fragrance and therapeutic properties are maintained. This preservation of the chemical nature is crucial for the quality and authenticity of the essential oils, making the adsorption method particularly valuable in industries where purity and scent fidelity are paramount.
In practical applications, the adsorption process can be finely tuned to optimize the recovery of specific types of volatile compounds. This adaptability allows for the extraction of a wide range of essential oils, each with its distinct chemical profile and aroma. By carefully selecting the adsorption medium and desorption conditions, it is possible to achieve high yields of essential oils that are both potent and pure.
Furthermore, the adsorption method is often preferred for its gentle handling of delicate plant materials. Unlike some other extraction techniques that may involve harsh chemicals or high temperatures, the adsorption method operates under conditions that minimize damage to the plant's volatile components. This gentle approach not only enhances the quality of the extracted oils but also extends the applicability of the method to a broader spectrum of aromatic plants.
In summary, the adsorption method offers a sophisticated and effective way to extract essential oils while preserving their original chemical properties and aromatic qualities. Its adaptability, efficiency, and gentle nature make it a valuable tool in the production of high-quality essential oils.
Press Method
The press method for extracting essential oils relies on mechanical compression to force the valuable ingredients out of plant cells. This technique typically operates under controlled conditions, often at room temperature or slightly lower, to preserve the integrity and quality of the extracted oils. Unlike other extraction methods that may involve heat or chemical solvents, the press method is a more straightforward and gentle approach, making it particularly suitable for delicate plant materials.
By applying mechanical pressure, the plant cells are ruptured, releasing their contents, including the essential oils. This process ensures that the oils are not subjected to high temperatures or harsh chemicals, which could alter their natural composition and aroma. The mechanical action involved in the press method is akin to squeezing a sponge, where the force applied is carefully calibrated to maximize oil yield without damaging the delicate aromatic compounds.

The press method is particularly favored for extracting oils from citrus peels, where the high pressure can efficiently release the volatile oils trapped within the fruit's outer layers. This method not only preserves the fresh, vibrant notes characteristic of citrus essential oils but also ensures that the oils retain their therapeutic properties, making them ideal for various applications in aromatherapy, culinary arts, and personal care products.
In summary, the press method offers a simple yet effective way to extract essential oils, leveraging mechanical pressure to gently coax the oils from plant cells while maintaining their natural qualities and aroma. This method stands out for its minimal processing and its ability to yield high-quality essential oils with minimal risk of degradation or contamination.
Supercritical Fluid Extraction Method
The supercritical fluid extraction (SFE) method leverages the unique properties of carbon dioxide (CO₂) when it is pressurized to a supercritical state. In this state, CO₂ exhibits both gas-like and liquid-like properties, allowing it to dissolve organic compounds effectively. This method is particularly advantageous because it leaves no solvent residue after extraction, making it a clean and efficient process.
Principle and Process
The principle behind SFE involves pressurizing CO₂ to a point where it transitions into a supercritical fluid. This fluid is then passed through a chamber containing the plant material, where it dissolves the desired compounds. The extract is subsequently separated from the CO₂ by reducing the pressure, which causes the CO₂ to evaporate, leaving behind a solvent-free plant extract.
Subcritical Extraction
In addition to supercritical extraction, the method also includes subcritical extraction, where CO₂ is pressurized to a state below supercritical but still above its critical point. This allows for greater control over the extraction process, enabling the isolation of specific components with varying solubility characteristics.
Temperature and Pressure Control
The SFE process is highly dependent on precise temperature and pressure control. Adjusting these parameters allows for the modification of the CO₂'s solubility, thereby influencing the profile of the extracted compounds. For instance, higher pressures and lower temperatures tend to enhance the extraction of less volatile compounds, while lower pressures and higher temperatures favor the extraction of more volatile ones.
Equipment and Safety
Sophisticated extraction apparatuses are employed in SFE, often incorporating refrigerated chillers and recirculating heaters to manage the CO₂'s state. These systems ensure that the CO₂ is efficiently recycled by condensing it back to a liquid state after extraction. The safety of the process is paramount, given the high pressures involved and the need to maintain CO₂ in its supercritical state.
Benefits and Applications
One of the significant advantages of SFE is its ability to yield extracts with a complete terpene profile, which is crucial for preserving the aroma and therapeutic properties of the plant material. This method is widely used in the cannabis industry, where it is favored for its ability to produce high-quality, solvent-free extracts.
Comparisons and Refinements
Applicability of Methods
Different extraction methods are tailored to various raw materials, each method aligning with the unique characteristics of the raw material, the nature of the components, and the specific process requirements. For instance, water vapor distillation is ideal for raw materials that are heat-stable and do not degrade under high temperatures, as it relies on the diffusion of essential oil components into water vapor. Conversely, extraction methods using volatile solvents like ethanol or benzene are more suitable for delicate or heat-sensitive materials, as they can extract aromatic components without subjecting the raw material to high temperatures.
The adsorption method is particularly effective for preserving the chemical nature of volatile components, making it a preferred choice for raw materials where the integrity of the aroma must be maintained. Pressing methods, on the other hand, are best suited for materials that can withstand mechanical compression, such as citrus peels, where the essential oils are readily accessible under pressure. Lastly, supercritical fluid extraction offers a solvent-free alternative, making it a green technology suitable for a wide range of raw materials, especially those requiring a high degree of purity and minimal chemical interaction.
In summary, the choice of extraction method is not arbitrary but is strategically selected to optimize the extraction process based on the inherent properties of the raw material, ensuring the highest quality and yield of essential oils.
Refinement of Essential Oils
The essential oils obtained through various extraction methods often contain a variety of impurities, including waxes, pigments, fats, and other unwanted substances. These impurities can affect the quality, purity, and overall efficacy of the essential oils, necessitating further refining processes to achieve the desired product.
Two primary methods are commonly employed for refining crude essential oils: fractional distillation or rectification, and molecular distillation.
-
Fractional Distillation: This method involves heating the crude oil to separate the components based on their boiling points. The process can be complex, requiring multiple stages to ensure the separation of different compounds.
-
Molecular Distillation: This is a more advanced technique, particularly suitable for heat-sensitive, high-boiling point products like essential oils. The process operates under very high vacuum and high temperature conditions, minimizing the exposure of the distillate to heat. The crude product is processed in a thin-film evaporator, where only the lightest components vaporize and condense almost immediately on the internal condenser. This results in two fractions: a "residue" containing heavy parts, pigments, and some pesticides, and a "distillate" containing the refined light parts.
The molecular distillation process is particularly advantageous as it ensures minimal thermal degradation of the essential oils, preserving their aromatic and therapeutic properties. This method is effective in removing impurities while maintaining the integrity of the essential oil components.
