Introduction: What is a Rotary Evaporator?
A rotary evaporator, or rotovap, is a laboratory equipment used for distillation of solvents and other compounds. It works by rotating the sample flask, while simultaneously heating it, to evaporate the solvent and separate it from the sample. The vapors are then condensed and collected in a separate flask. The rotary evaporator is commonly used in chemistry, biology, and pharmaceuticals for the purification and concentration of samples. It is also used for solvent recycling, reducing waste and saving costs.
Table of Contents
Advantages of Rotary Evaporators:
Rotary evaporators have become an essential tool in modern laboratories across various industries. They offer several advantages over traditional distillation equipment, making them the superior choice for separating and purifying chemical compounds from solvents.

Lower Boiling Temperatures
Rotary evaporators are used together with a vacuum system to reduce the pressure of the system. This means that the separation of solvents and other compounds can be achieved at lower temperatures than usual. Lower water bath temperatures ensure that the apparatus stays in good shape for longer as they are exposed to lower temperatures.
Faster Evaporation of Solvent
The process of separation in rotary evaporators involves two main forces: the centripetal force and friction. This allows the mixture to form a film on the inner surface of the flask, creating a larger surface area for heating. The rotation of the flask at a constant speed increases surface area. Under the low pressure caused by the vacuum pump, the distillation speed is increased significantly.
Fewer Operations
A rotary evaporator consists of a built-in lifting and falling motor. This part enables the automatic raising of the rotary bottle to a position just above the water bath for the rotation process. The motor is electrically powered, and this means that the apparatus can be used so long as there is power. This makes it an efficient and reliable apparatus for most Chemistry labs.
Rotary Evaporators Suppress Bumping
Due to the forces that contribute to the evaporation process (centripetal force and friction), bumping is often suppressed. This allows for quick and gentle evaporation of mixtures, and therefore allows even the inexperienced users to utilize these apparatus. The solvent remaining after the rotary evaporation can further be removed through a deeper vacuum or a tightly sealed vacuum system at ambient or relatively higher temperatures.
Versatility
Rotary evaporators offer precise temperature control and allow for the use of a wide range of solvents. This versatility makes them suitable for a variety of applications, from pharmaceuticals to food processing.
Efficient Solvent Recovery
Another advantage of rotary evaporators is their efficiency in recovering solvents. They can recover up to 90% of the solvent used in the process, making them a cost-effective solution.
In conclusion, the above advantages make rotary evaporators an essential tool for laboratories looking to scale up their sample processing capabilities while improving efficiency and maintaining high standards of purity.
Disadvantages of Rotary Evaporators:

Cost
Rotary evaporators can be quite costly, depending on the brand and model. The initial investment required to purchase a rotary evaporator can be a significant expenditure for some labs. However, it is important to note that the cost can be offset by the efficiency and accuracy of the results obtained using a rotary evaporator.
Maintenance and Calibration
Another disadvantage of rotary evaporators is the need for regular maintenance and calibration to ensure accurate results. The equipment must be checked frequently to maintain the quality of the results obtained. In addition, regular calibration of the equipment is necessary to ensure that the results obtained are accurate and consistent. Failure to perform regular maintenance and calibration can result in inaccurate results and equipment failure.
Operator Skill
Rotary evaporators can be time-consuming and require a skilled operator to operate. The operator must have a good understanding of the equipment and its operation to ensure that the results obtained are accurate and consistent. This requires a certain level of training and experience, which can be a challenge for some labs.
Solvent Bumping
Rotary evaporators are prone to solvent bumping, which can result in the loss of samples and inaccurate results. Solvent bumping occurs when the solvent boils too rapidly and causes the sample to bump into the condenser. This can be prevented by adjusting the vacuum pressure and temperature settings, but it requires skilled operation and precise adjustments.
Fragile Glassware
Rotary evaporators are also prone to breakage due to the fragile nature of the glassware used in the equipment. The glassware is delicate and can be easily damaged, resulting in costly repairs or replacements. It is important to handle the glassware with care and avoid any sudden movements or impacts that could cause it to break.
Overall, despite their disadvantages, rotary evaporators are still a valuable asset in the laboratory. With proper use, maintenance, and operator training, they can help researchers and scientists to achieve accurate and efficient results in their work.
Functions of Rotary Evaporators:
A rotary evaporator is an essential separation equipment that can remove a volatile solvent from a liquid mixture through evaporation and condensation. The device has various functions, including:
Concentration
A rotary evaporator is an ideal tool for concentrating non-volatile components in a mixture. It enables the concentration of purest and freshest flavors from a blood orange by removing water. It can also concentrate the volatile aroma and flavor molecules from mixtures gently and at low temperatures. For example, it can extract the desired flavors from a blend of alcohol, herbs, and fruit without heating the mixture up.
Extraction
A rotary evaporator is useful for extracting volatile compounds from samples without altering them. It can separate food compounds from one another without altering them, unlike conventional distilling apparatuses. For instance, the device can be used to remove ethanol from the mixture of ethanol and CBD oil to improve the purity of CBD oil. It can also remove water within fruit juice, making the fruit juice have a higher concentration.
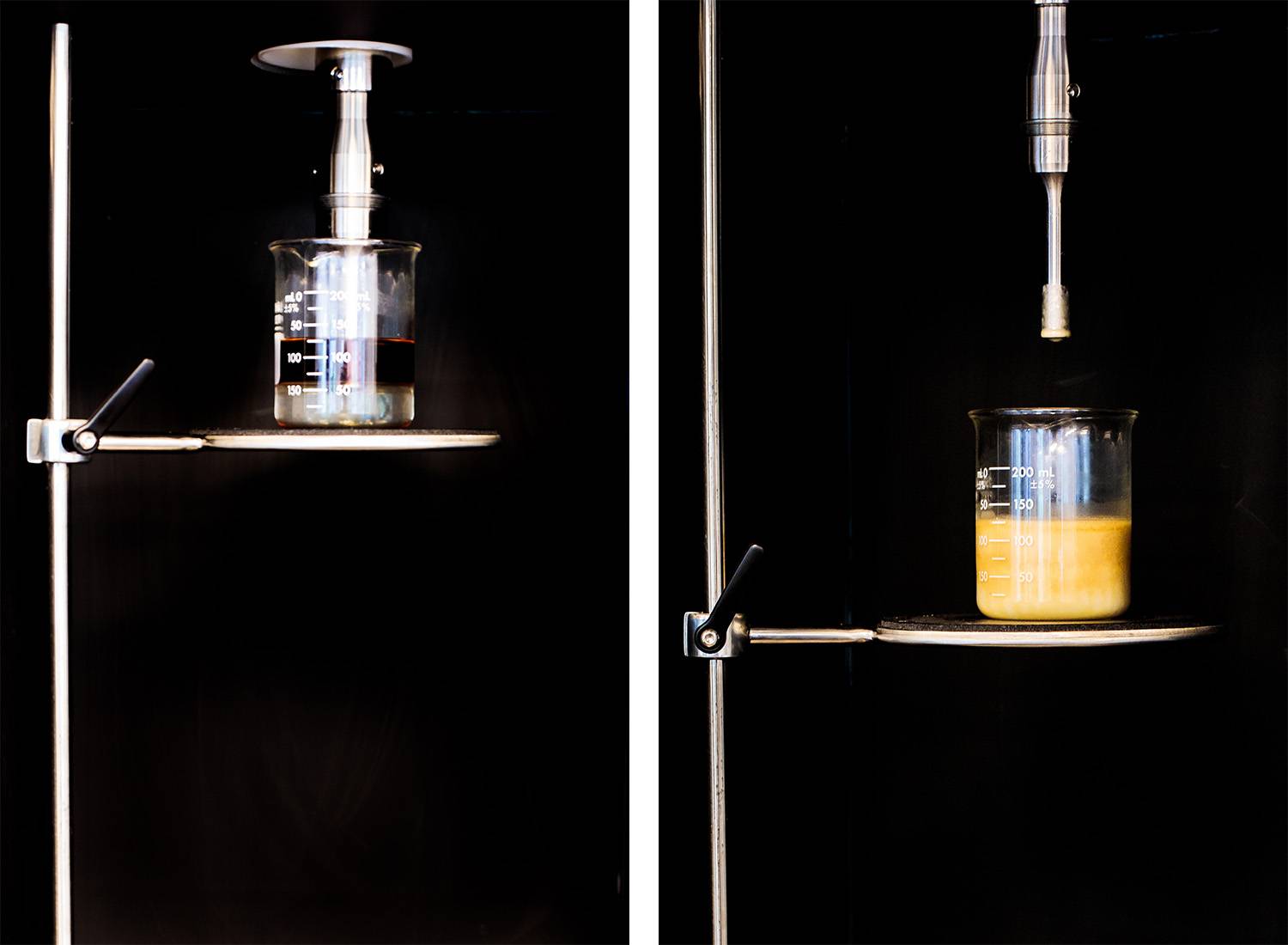
Purification
A rotary evaporator can purify biological products that are easily decomposed and denatured at high temperature. It is especially suitable for the concentration and purification of biological products. The rotating flask is heated thermostatically and can rotate at a constant speed. The material forms a large area of a thin film on the inner surface of the flask, and the solvent vapor is recycled in the collecting bottle after being cooled by the high-efficiency glass condenser, which greatly improves the evaporation efficiency.
Drying
A rotary evaporator is effective in drying liquid samples. The rotating flask is heated at a constant temperature, and the material forms a large-area film on the bottle wall and evaporates. The solvent vapor is cooled by the glass condenser and recovered in the collection bottle, which greatly improves the evaporation efficiency.
Solvent recovery
A rotary evaporator is essential in the solvent recovery process. It can recover almost 100% of the solvent. The device is mainly used for drying, concentration, crystallization, separation, and solvent recovery in chemical, pharmaceutical, and biopharmaceutical industries.
In conclusion, the rotary evaporator is a highly useful device in the distillation processes of cannabis extraction, chemical labs, and biotechnology industries. Its efficiency in solvent removal, gentle extraction of volatile compounds, and purification of biological products makes it a versatile and essential tool in many laboratory applications. By understanding the functions of rotary evaporators, researchers can better utilize them in their work and achieve more precise and efficient results.
Factors Affecting Distillation Efficiency:
Temperature of the Heating Bath
- Higher temperature of the bath increases the rate of evaporation
- Boiling temperature of the product is determined by the pressure of the system
- In a rotovap, the temperature of the heat input is kept constant and the distillation is adjusted by controlling the pressure
Pressure of the Distillation
- Lower pressure decreases the boiling point of the solvent, leading to faster distillation at a given bath temperature
- The presence of a vacuum decreases the pressure within the system, thus reducing the vapor pressure required for boiling to occur
Speed of Rotation and Size of the Flask
- Larger flask and higher RPM increase the surface area exposed per unit time, leading to quicker evaporation
- The faster the motor speed, the larger the area of the evaporation bottle, and the higher the distillation efficiency

Size and Power of the System
- The speed of distillation is limited primarily by the condenser’s ability to condense the solvent vapor
- The incoming vapor must not overload the condenser; otherwise, valuable volatiles may be lost
- To avoid saturation, the pressure of the system should be monitored such that 2/3 of the condenser is consistently covered with condensate
In conclusion, various factors affect the efficiency of distillation in rotary evaporators, including the temperature of the heating bath, pressure of the distillation, speed of rotation and size of the flask, and size and power of the system. Scientists can achieve optimal distillation efficiency by controlling these variables. Rotary evaporators are safer and more efficient than traditional distillation methods, making them an indispensable tool in many laboratory processes.
How Does a Rotovap Work?
A rotary evaporator or rotovap is an essential tool in a processing facility. Its primary use is in the recovery of ethanol after a winterization or extraction process. Although we’ll be focusing on how a rotovap operates during a basic ethanol recovery, know that it can be applied in the recovery of many other solvents that are compatible with the gasket materials.
Understanding Rotovap Distillation
Before learning about rotovap setup and operation, you must have a clear picture of the physical change happening inside of the rotary evaporator. Consider how a mixture containing extract and ethanol must first be separated before rotovap distillation can occur.
To separate the mixture, heat is applied while simultaneously pulling your vacuum. This significantly drops the boiling point of the ethanol. As the evaporation flask turns, the mixture creates a thin film that spreads across the interior of the flask. This helps force the ethanol to vaporize at a faster rate. That vapor is pulled into the path of the condenser by the vacuum pump.
Components Of A Rotovap
Before you learn how to successfully use a rotovap for evaporation, you should understand what components make it up. You can refer to our rotovap turnkey setups for specific component combinations.

Rotovap
The rotovap size depends on how many liters you need to recover. That being said, the most common sizes are 5L, 10L, 20L, and 50L. The size refers to the size of the evaporating flask. It’s important to note that the flask is not loaded at max capacity during operation, but usually around 20-40% capacity.
Chiller
Rotovap chillers are specially designed. They have a much higher cooling capacity compared to refrigerated circulators. However, they usually don’t have as large of a temperature range. That’s because you won’t need extremely cold temperatures for evaporation: just a higher cooling capacity. The solvent vapor is warm when it hits the condenser. The increased cooling capacity is meant to keep your chiller at a stable temperature.
Vacuum Pump
For best results, a chemical-resistant, oil-less diaphragm pump should be used with your rotovap. These pumps don’t have as much depth as rotary vane (oil) pumps but they are much more resistant to contamination. Don’t use an oil pump with a rotovap or you’ll find it destroyed in no time.
Rotary Evaporator Working Principle
The rotatory evaporator working principle is that the boiling point of liquids lowers on decreasing their pressure. This allows the solvent to vaporize at lower temperatures than when boiled in a normal atmosphere. You should choose a vacuum pump for your rotavap that will guarantee an improved evaporation efficiency.
Rotatory evaporation is mostly considered when separating low boiling solvents such as ethyl acetate or n-hexane from compounds that are solid at room temperatures. When carefully monitored, one can remove a solvent from a sample with a liquid compound. Solvents with much higher boiling points such as water can also be evaporated if the instrument can withstand very low pressures.
The condenser condenses the gas into a liquid by chilling and cooling the vapor. To achieve these low temperatures a number of things can be used including liquid nitrogen, cold water, and dry ice. The evaporation vessel is constantly rotating so as to increase the evaporation surface area per unit time. The centrifugal force produced as a result of the rotation keeps the liquid stuck to the inside of the vessel providing a large surface area and accelerating the evaporation process.
There is a 3-way piston between the pressure reducing pump and the condenser tube. When the operating system is connected with the pressure, the evaporation flask is removed to obtain the residue.
Related Products
Related Articles
- A Comprehensive Guide to Understanding Rotary Evaporator Chillers
- Common Mistakes to Avoid When Using a Rotary Vacuum Evaporator
- Installation of Tube Furnace Fitting Tee
- Exploring the Science Behind Rotary Evaporators: How They Work and Their Applications
- Boost Your Efficiency Why A Rotary Evaporator Is Better

