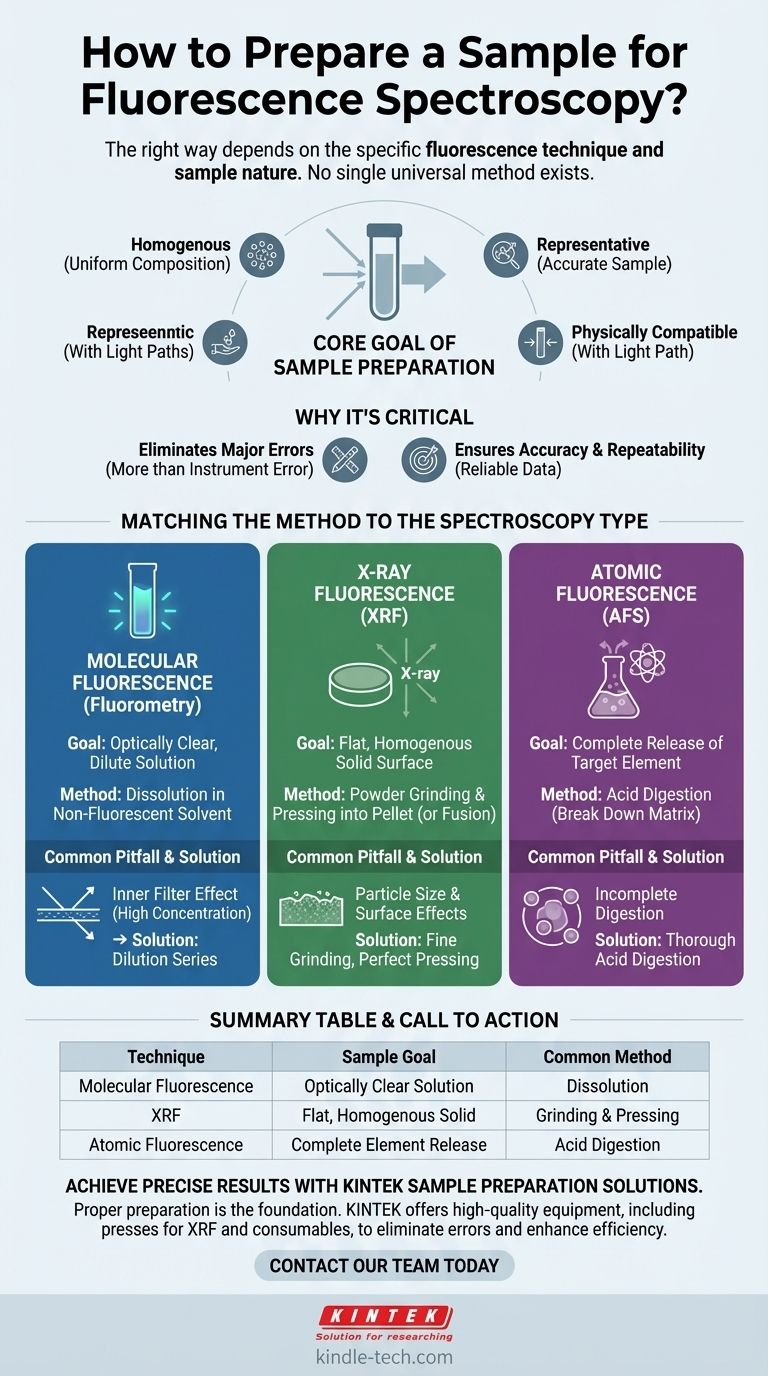
The right way to prepare a sample for fluorescence spectroscopy depends entirely on the specific type of fluorescence technique you are using and the nature of your sample. There is no single universal method; sample preparation for analyzing a dissolved molecule (molecular fluorescence) is completely different from preparing a solid rock for elemental analysis (X-ray fluorescence) or a water sample for mercury detection (atomic fluorescence).
The core goal of any sample preparation is to transform your material into a form that is homogenous, representative, and physically compatible with your instrument's light path to ensure an accurate and repeatable measurement.

Why Sample Preparation is the Most Critical Step
It is a common mistake to focus only on the instrument's sophistication while overlooking the preparation process. However, the uncertainty and error introduced by poor sample preparation can be far greater than any instrumental error.
The Source of Major Errors
Incorrect preparation becomes a primary source of analytical error. If the sample presented to the instrument does not accurately represent the original material, the resulting data, no matter how precise, will be inaccurate.
The Principle of Homogeneity
The fundamental goal is to eliminate variability within the sample. Whether it's a liquid or a solid, the portion being measured must be identical to any other portion, ensuring the result is reliable and representative of the whole.
Matching the Method to the Spectroscopy Type
The physical state required for your sample is dictated by the physics of the technique. The three main branches of fluorescence spectroscopy demand radically different approaches.
For Molecular Fluorescence (Fluorometry)
This is the most common technique, used for analyzing fluorescent molecules, dyes, or proteins in a solution.
The goal is to create an optically clear, dilute solution. The sample is typically held in a quartz or glass cuvette. Key considerations are concentration (to avoid inner filter effects) and choosing a non-fluorescent solvent that won't interfere with the measurement.
For X-ray Fluorescence (XRF)
This technique is used to determine the elemental composition of a sample, which is often a solid.
The purpose of preparation is to create a sample with a uniform composition and a perfectly flat surface. Common methods include grinding a powder and pressing it into a dense pellet or fusing the powder with a flux (like lithium borate) to create a homogenous glass disc.
For Atomic Fluorescence (AFS)
This technique is used to quantify specific elements, often trace metals like mercury.
The sample must be completely broken down to release the target element as free atoms. This is typically achieved through acid digestion, where strong acids dissolve the sample matrix, ensuring all the mercury (or other target element) is available for measurement.
Common Pitfalls and How to Avoid Them
Even with the correct general approach, subtle mistakes can invalidate your results. Understanding these trade-offs is key to generating trustworthy data.
The "Inner Filter Effect" in Solutions
For molecular fluorescence, if your sample concentration is too high, the emitted light can be re-absorbed by other molecules in the solution before it reaches the detector. This leads to a non-linear response and an underestimation of the true fluorescence. Always perform a dilution series to find the optimal concentration range.
Particle Size and Surface Effects in Solids
For XRF, if a powder sample is not ground finely enough, large particles can cause inconsistent X-ray scattering and absorption, skewing the results. Similarly, any imperfections, cracks, or unevenness on the surface of a pressed pellet will lead to erroneous readings.
Incomplete Digestion for Elements
For AFS, if the acid digestion is incomplete, some of the target element will remain trapped in the sample matrix and will not be atomized and measured. This directly leads to an underestimation of the element's concentration.
Making the Right Choice for Your Goal
To ensure accurate results, align your preparation strategy with your analytical objective.
- If your primary focus is analyzing a dissolved molecule (like a fluorescent dye or protein): Your goal is to prepare a dilute, optically clear solution in a non-interfering solvent.
- If your primary focus is determining the elemental composition of a solid (like a rock or polymer): Your goal is to create a perfectly flat and homogenous solid surface, typically by pressing a fine powder into a pellet or fusing it into a glass disc.
- If your primary focus is quantifying a specific trace element (like mercury in water): Your goal is to completely digest the sample, usually with acid, to release all atoms of the target element into a solution.
Ultimately, successful fluorescence spectroscopy begins with a meticulously prepared sample that is perfectly suited for your specific instrument and analytical question.
Summary Table:
| Technique | Sample Goal | Common Preparation Method |
|---|---|---|
| Molecular Fluorescence | Optically clear, dilute solution | Dissolution in non-fluorescent solvent |
| X-ray Fluorescence (XRF) | Flat, homogenous solid surface | Powder grinding & pressing into a pellet |
| Atomic Fluorescence | Complete release of target element | Acid digestion |
Achieve precise and reliable fluorescence results with sample preparation solutions from KINTEK.
Proper sample preparation is the foundation of accurate data. Whether you are pressing pellets for XRF analysis or preparing solutions for molecular fluorescence, using the right equipment is critical. KINTEK specializes in high-quality lab equipment, including presses for creating perfect XRF pellets and consumables for all your preparation needs.
Let us help you eliminate preparation errors and enhance your lab's efficiency. Our experts can guide you to the ideal solution for your specific application.
Contact our team today to discuss your requirements and ensure your samples are prepared for success!
Visual Guide
Related Products
- Custom PTFE Teflon Parts Manufacturer for PTFE Measuring Cylinder 10/50/100ml
- Optical Window Glass Substrate Wafer Barium Fluoride BaF2 Substrate Window
- Optical Window Glass Substrate Wafer CaF2 Substrate Window Lens
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Laboratory Multifunctional Small Speed-Adjustable Horizontal Mechanical Shaker for Lab
People Also Ask
- What are the four main types of sensors? A Guide to Power Source and Signal Type
- Why is a PTFE Mold Selected for Preparing Composite Films? Ensure Pure, Damage-Free Results
- What are the advantages of using PTFE jars for RuTi alloy mixing? Ensure Chemical Purity and High Yield
- Why are PTFE beakers required for hafnium metal ICP-OES validation? Ensure Pure Sample Dissolution
- Why is slender PTFE tubing necessary for flow control in multi-channel catalyst aging? Ensure Equal Gas Distribution

















