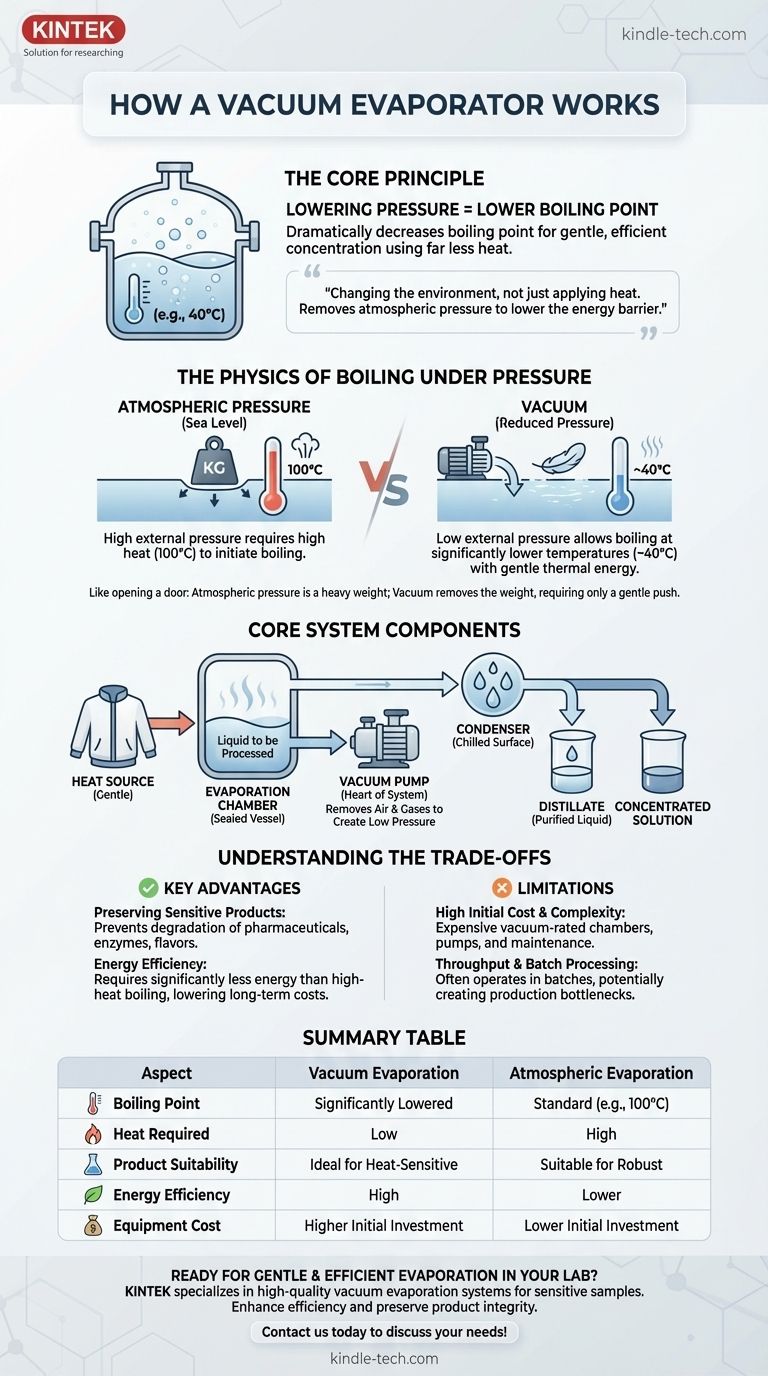
At its core, a vacuum evaporator works by lowering the pressure within a sealed chamber. This pressure reduction dramatically decreases the boiling point of the liquid inside. As a result, the liquid can be turned into a vapor using far less heat than would be required at normal atmospheric pressure, allowing for gentle and efficient concentration or separation.
The fundamental principle is not about applying more heat, but about changing the environment. By removing atmospheric pressure, you lower the energy barrier for a liquid to become a gas, enabling evaporation without the high temperatures that could damage the product.

The Physics of Boiling Under Pressure
To understand vacuum evaporation, you first need to re-examine what "boiling" actually is. It’s a battle between the liquid's internal vapor pressure and the external atmospheric pressure.
What Defines Boiling?
Boiling occurs when a liquid's vapor pressure—the pressure exerted by its vapor—becomes equal to the pressure of the surrounding environment. At sea level, atmospheric pressure is high, so water must be heated to 100°C (212°F) to generate enough vapor pressure to boil.
The Role of the Vacuum
A vacuum pump is used to actively remove air molecules from a sealed evaporation chamber. This action significantly lowers the environmental pressure pushing down on the surface of the liquid.
Lower Pressure Equals a Lower Boiling Point
With very little external pressure, the liquid needs far less thermal energy for its vapor pressure to win the battle and initiate boiling. It's like trying to open a heavy door; atmospheric pressure is the weight pushing against it. In a vacuum, that weight is removed, and the door opens with a gentle push.
The Core Components of the System
A vacuum evaporator is a system of interconnected parts, each with a critical function. Understanding these components helps clarify the entire process.
The Evaporation Chamber
This is the sealed vessel that holds the liquid to be processed. It must be robust enough to withstand the immense force of the outside atmosphere pushing inward when a vacuum is created inside.
The Vacuum Pump
This is the heart of the system. Its sole job is to remove air and other non-condensable gases from the chamber to create and maintain the low-pressure environment required for the process.
The Heat Source
Even at a lower boiling point, some energy is still needed to trigger the phase change from liquid to vapor. This is provided by a heat source, often a heating jacket around the chamber or internal steam coils, which gently raises the liquid's temperature.
The Condenser and Collection
Once the vapor is created, it moves to a separate section containing a condenser. The condenser is a chilled surface that causes the vapor to rapidly cool and turn back into a pure liquid (called the distillate). This purified liquid is then collected in a separate vessel, leaving the original, now-concentrated solution behind.
Understanding the Trade-offs
While powerful, vacuum evaporation is not a universal solution. Its benefits come with specific costs and limitations.
Key Advantage: Preserving Sensitive Products
This is the primary reason for using vacuum evaporation. Lower temperatures prevent the degradation of heat-sensitive materials. This is essential for concentrating pharmaceuticals, fruit juices, milk, and extracting delicate essential oils where high heat would destroy the product's quality and efficacy.
Key Advantage: Energy Efficiency
Heating a substance to 40°C requires significantly less energy than heating it to 100°C. Over time, especially in large-scale industrial operations, this can lead to substantial reductions in energy consumption and operational costs.
Limitation: High Initial Cost and Complexity
Vacuum-rated chambers, high-performance pumps, and the associated control systems are significantly more expensive than simple atmospheric boiling tanks. The equipment also requires specialized maintenance to ensure seals are tight and the vacuum is held.
Limitation: Throughput and Batch Processing
Many vacuum evaporators operate in batches—a single load is processed, the system is cleaned, and a new load is introduced. This can create a bottleneck in a continuous production line, though more complex and expensive continuous systems do exist.
Making the Right Choice for Your Goal
Selecting an evaporation method depends entirely on the nature of your product and your primary operational objective.
- If your primary focus is preserving product quality: Vacuum evaporation is non-negotiable for heat-sensitive materials like pharmaceuticals, enzymes, or delicate flavor compounds.
- If your primary focus is energy efficiency at scale: This method can offer significant long-term cost savings by reducing the thermal energy required for evaporation.
- If your primary focus is simple separation of robust liquids: A standard atmospheric evaporator may be a more cost-effective and simpler solution if your product is not damaged by high temperatures.
Ultimately, leveraging a vacuum transforms evaporation from a brute-force heating process into a precise and gentle method of separation.
Summary Table:
| Aspect | Vacuum Evaporation | Atmospheric Evaporation |
|---|---|---|
| Boiling Point | Significantly Lowered | Standard (e.g., 100°C for water) |
| Heat Required | Low | High |
| Product Suitability | Ideal for heat-sensitive materials | Suitable for robust materials |
| Energy Efficiency | High | Lower |
| Equipment Cost | Higher initial investment | Lower initial investment |
Ready to implement a gentle and efficient evaporation process in your lab?
KINTEK specializes in providing high-quality lab equipment, including vacuum evaporation systems, to help you concentrate sensitive samples like pharmaceuticals, enzymes, and food extracts without degradation. Our solutions are designed to enhance your lab's efficiency while preserving product integrity.
Contact us today to discuss your specific needs and discover how our expertise can benefit your laboratory!
Visual Guide
Related Products
- Circulating Water Vacuum Pump for Laboratory and Industrial Use
- Molybdenum Tungsten Tantalum Evaporation Boat for High Temperature Applications
- Oil Free Diaphragm Vacuum Pump for Laboratory and Industrial Use
- Ceramic Evaporation Boat Set Alumina Crucible for Laboratory Use
- Molybdenum Tungsten Tantalum Special Shape Evaporation Boat
People Also Ask
- What is the purpose of the compression chamber in a vacuum pump? The Heart of Vacuum Generation
- What can I use a vacuum pump for? Powering Industrial Processes from Packaging to Automation
- How does a water circulating vacuum pump operate? Discover the Efficient Liquid Piston Principle
- What determines the vacuum degree achievable by a water circulating vacuum pump? Unlock the Physics of Its Limits
- Why is a water circulating vacuum pump suitable for handling flammable or explosive gases? Inherent Safety Through Isothermal Compression



















