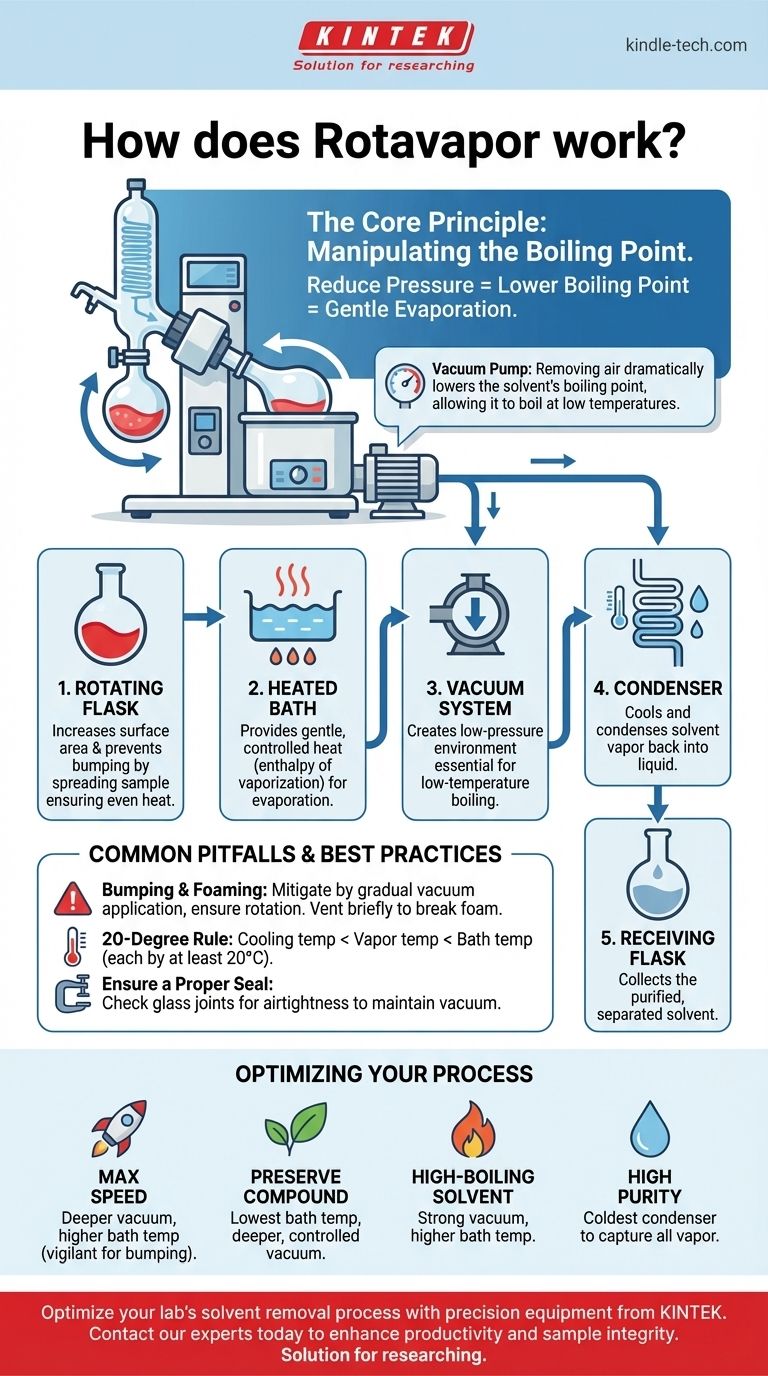
At its core, a rotary evaporator, or Rotavapor, separates solutes from solvents through a gentle evaporation process. It accomplishes this by reducing the pressure within the system, which lowers the solvent's boiling point, while simultaneously rotating the sample to increase its surface area and prevent violent boiling. This allows for fast and efficient solvent removal at a low temperature, preserving the integrity of the sample.
The central principle of a Rotavapor is not about heating a solvent until it boils at atmospheric pressure, but rather about lowering the pressure until the solvent boils at a gentle, controlled temperature.

The Core Principle: Manipulating the Boiling Point
A substance's boiling point is not a fixed number; it is a function of the pressure surrounding it. A Rotavapor is designed to exploit this physical law for efficient and gentle solvent removal.
What Determines a Boiling Point?
Think of the pressure of the atmosphere as a weight pushing down on the surface of a liquid. For a liquid to boil, its own vapor pressure must become strong enough to overcome this atmospheric weight.
Heating a liquid gives its molecules more energy, increasing their vapor pressure until it matches the surrounding pressure, at which point boiling begins.
The Role of Reduced Pressure (Vacuum)
The vacuum pump is the most critical component. By removing air from the system, it dramatically reduces the "weight" pressing down on the solvent.
With less external pressure, the solvent's vapor pressure doesn't need to be nearly as high to initiate boiling. This means the solvent will boil at a much lower temperature than it normally would.
The Importance of Gentle Heat
The heated water bath provides the necessary energy (enthalpy of vaporization) to turn the liquid solvent into a gas.
Because the vacuum has already lowered the boiling point, the water bath only needs to be lukewarm. This gentle heating is crucial for preserving temperature-sensitive compounds that would be destroyed by traditional distillation.
A Breakdown of the Key Components
Each part of the Rotavapor plays a distinct role in controlling the interplay of pressure, temperature, and surface area.
The Rotating Flask
The rotation of the sample flask serves two purposes. First, it constantly spreads the sample into a thin film on the inner wall of the flask, dramatically increasing the surface area for evaporation.
Second, this rotation ensures even heat distribution and agitation, which prevents bumping—the sudden, violent boiling that can cause sample loss.
The Vacuum System
The vacuum pump actively removes air and solvent vapor from the sealed apparatus. This creates and maintains the low-pressure environment essential for low-temperature boiling.
The Condenser
As the solvent evaporates in the rotating flask, its vapor travels into the condenser. The condenser contains a cold coil, typically chilled with circulating water or a coolant.
When the warm solvent vapor hits this cold surface, it rapidly condenses back into a liquid.
The Receiving Flask
This is the final destination for the purified solvent. The condensed liquid drips from the condenser coils and collects in this stationary flask, effectively separated from the original solute.
Common Pitfalls and Best Practices
While highly effective, achieving optimal results with a Rotavapor requires understanding potential issues and proper technique.
The Risk of Bumping and Foaming
Bumping occurs when a solution superheats and boils explosively. This is mitigated by the flask's rotation, but can still occur if the vacuum is applied too quickly or the temperature is too high. Always apply vacuum gradually.
Foaming is also a common issue. If your sample begins to foam into the condenser, you can briefly and gently vent the system to break the vacuum, which will cause the foam to collapse.
Choosing the Right Parameters
The efficiency of the process depends on balancing bath temperature and vacuum depth. A general rule of thumb is the "20-degree rule": the cooling temperature should be at least 20°C lower than the vapor temperature, which should be at least 20°C lower than the heating bath temperature. Using a nomograph chart can help you find the ideal pressure for a given solvent and temperature.
Ensuring a Proper Seal
The entire system relies on being airtight. The most common cause of poor performance is a vacuum leak. Ensure all glass joints are clean, properly greased (if necessary), and securely clamped to maintain the target pressure.
Optimizing Your Evaporation Process
Your specific goal will determine the ideal settings for your rotary evaporation.
- If your primary focus is maximum speed: Use a deeper vacuum and a slightly higher bath temperature, but be vigilant for bumping and foaming.
- If your primary focus is preserving a fragile compound: Prioritize the lowest possible bath temperature by pulling a deeper, more controlled vacuum.
- If you are working with a high-boiling-point solvent: You will require both a strong vacuum and a higher bath temperature to achieve efficient evaporation.
- If you need to re-collect the solvent with high purity: Ensure your condenser is sufficiently cold to capture all the vapor and prevent any loss through the vacuum pump.
By mastering the relationship between pressure, temperature, and surface area, you can precisely control the separation process for nearly any application.
Summary Table:
| Component | Key Function |
|---|---|
| Vacuum Pump | Lowers pressure to reduce solvent boiling point |
| Rotating Flask | Increases surface area and prevents bumping |
| Heated Bath | Provides gentle, controlled heat for evaporation |
| Condenser | Cools and condenses solvent vapor back to liquid |
| Receiving Flask | Collects the purified, separated solvent |
Optimize your lab's solvent removal process with precision equipment from KINTEK.
Whether you are working with temperature-sensitive compounds or high-boiling-point solvents, the right rotary evaporator is key to efficient and gentle separations. KINTEK specializes in high-quality lab equipment and consumables, providing reliable solutions for all your laboratory needs.
Contact our experts today to find the perfect Rotavapor system for your application and enhance your lab's productivity and sample integrity.
Visual Guide
Related Products
- Circulating Water Vacuum Pump for Laboratory and Industrial Use
- Oil Free Diaphragm Vacuum Pump for Laboratory and Industrial Use
- 30T 40T Split Automatic Heated Hydraulic Press Machine with Heated Plates for Laboratory Hot Press
- Electric Heated Hydraulic Vacuum Heat Press for Lab
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- How does the impeller rotation affect the gas flow in a water circulating vacuum pump? A Guide to the Liquid Ring Principle
- What determines the vacuum degree achievable by a water circulating vacuum pump? Unlock the Physics of Its Limits
- Why is a water circulating vacuum pump suitable for handling flammable or explosive gases? Inherent Safety Through Isothermal Compression
- How does a water circulating vacuum pump operate? Discover the Efficient Liquid Piston Principle
- What can I use a vacuum pump for? Powering Industrial Processes from Packaging to Automation



















