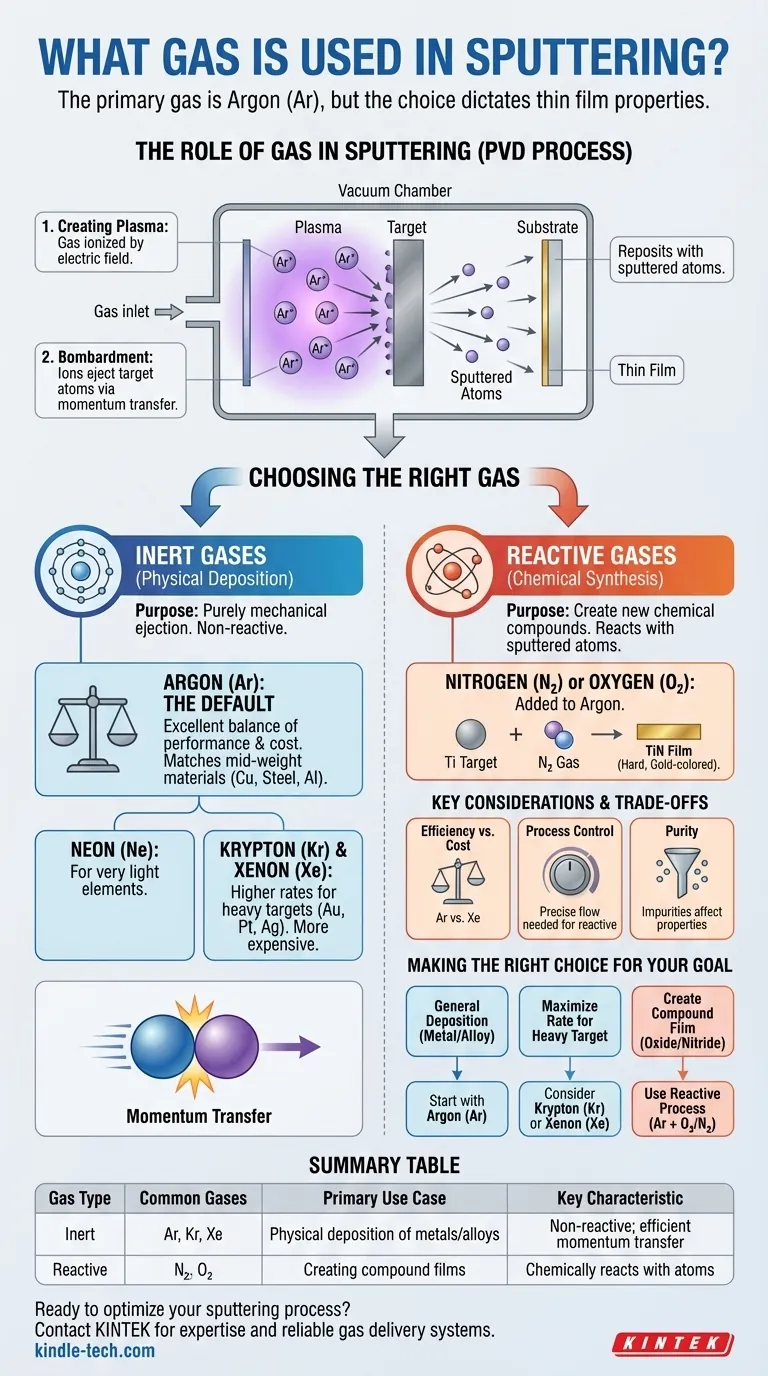
The primary gas used in sputtering is Argon (Ar). As an inert noble gas, Argon provides the ideal combination of atomic mass, cost-effectiveness, and chemical stability needed to physically eject atoms from a target material without reacting with them. While Argon is the default choice, the specific gas used is a critical process parameter tailored to the desired outcome.
The core principle is this: sputtering requires a gas to create a plasma and bombard a target. The choice between an inert gas like Argon for purely physical deposition and a reactive gas like Nitrogen for chemical synthesis is the fundamental decision that dictates the final properties of your thin film.

The Role of Gas in Sputtering
Sputtering is a physical vapor deposition (PVD) process. The gas does not simply create an atmosphere; it is an active and essential component of the deposition mechanism.
Creating the Plasma
The process begins by introducing a low-pressure gas into a vacuum chamber. A strong electric field is then applied, which ionizes the gas atoms by stripping them of electrons. This creates a glowing, energized state of matter known as plasma, consisting of positive ions and free electrons.
The Bombardment Process
The positively charged gas ions within the plasma are accelerated by the electric field and directed with high energy toward the "target," which is the source material you wish to deposit.
Think of it as a subatomic game of pool. The gas ions are the cue balls, and the atoms of the target material are the object balls. Upon impact, the momentum from the gas ions is transferred to the target atoms, ejecting, or "sputtering," them from the surface. These ejected atoms then travel through the chamber and deposit onto a substrate, forming a thin film.
Choosing the Right Sputtering Gas
The selection of a sputtering gas is a deliberate choice between two distinct categories: inert gases for physical deposition and reactive gases for creating new chemical compounds.
Inert Gases: The Physical Workhorse
Inert gases (also called noble gases) are used because they are chemically non-reactive. Their purpose is purely mechanical: to physically dislodge atoms from the target.
The key factor for selecting an inert gas is achieving efficient momentum transfer. For the most effective "knock-out" of target atoms, the atomic weight of the sputtering gas should be as close as possible to the atomic weight of the target material.
Why Argon Is the Default
Argon is the most common sputtering gas because it offers an excellent balance of performance, availability, and cost. Its atomic mass (39.95 u) is a suitable match for many commonly sputtered mid-weight materials like copper, steel, and aluminum.
Matching Gas to Target Weight
For more specialized applications, other inert gases are used:
- Neon (Ne): With a lower atomic mass, Neon is more effective for sputtering very light elements.
- Krypton (Kr) & Xenon (Xe): These heavier, more expensive gases provide significantly higher sputtering rates for heavy target materials like gold, platinum, or silver due to their superior momentum transfer.
Reactive Gases: For Chemical Synthesis
In a process known as reactive sputtering, a reactive gas like nitrogen (N₂) or oxygen (O₂) is intentionally added to the inert Argon atmosphere.
These gases react with the sputtered target atoms as they travel toward the substrate. This allows for the deposition of compound thin films that are different from the source target. For example, you can sputter a pure titanium target in a nitrogen atmosphere to create a hard, gold-colored titanium nitride (TiN) film on the substrate.
Understanding the Trade-offs
Choosing a gas involves balancing efficiency, cost, and process complexity. There is no single "best" gas for all situations.
Efficiency vs. Cost
While Xenon offers the highest sputter yield for heavy materials, it is substantially more expensive than Argon. For most applications, the increased deposition rate from using Krypton or Xenon does not justify the significant increase in operational cost compared to using Argon.
Process Control in Reactive Sputtering
Reactive sputtering is a powerful technique, but it adds a layer of complexity. The flow rate of the reactive gas must be precisely controlled. Too little gas results in an incomplete reaction, while too much can lead to "poisoning," where the reactive gas forms a compound layer on the target itself, drastically reducing the sputter rate.
Purity and Contamination
The purity of the sputtering gas is paramount. Even small amounts of impurities, such as water vapor or oxygen in an inert gas system, can become incorporated into the growing film, negatively affecting its electrical, optical, or mechanical properties.
Making the Right Choice for Your Goal
Your choice of gas should be directly driven by the material you want to create.
- If your primary focus is general-purpose deposition of a metal or alloy: Start with Argon (Ar), as it provides the best balance of cost and performance for a wide range of materials.
- If your primary focus is maximizing the deposition rate for a heavy target (e.g., gold): Consider Krypton (Kr) or Xenon (Xe), but only if the higher throughput justifies the significant increase in gas cost.
- If your primary focus is creating a compound film (e.g., an oxide or nitride): You must use a reactive sputtering process, blending a reactive gas like oxygen (O₂) or nitrogen (N₂) with your primary inert gas, Argon.
Understanding these gas selection principles is the key to controlling the composition and properties of your deposited thin film.
Summary Table:
| Gas Type | Common Gases | Primary Use Case | Key Characteristic |
|---|---|---|---|
| Inert | Argon (Ar), Krypton (Kr), Xenon (Xe) | Physical deposition of metals/alloys | Non-reactive; efficient momentum transfer |
| Reactive | Nitrogen (N₂), Oxygen (O₂) | Creating compound films (e.g., nitrides, oxides) | Chemically reacts with sputtered atoms |
Ready to optimize your sputtering process? The right gas selection is critical for achieving the desired thin film properties, from pure metals to advanced compounds. KINTEK specializes in lab equipment and consumables, providing the expertise and reliable gas delivery systems your laboratory needs for precise and contamination-free deposition. Contact our experts today to discuss your specific application and ensure optimal results.
Visual Guide
Related Products
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- Custom PTFE Teflon Parts Manufacturer for Acid and Alkali Resistant Chemical Powder Material Scoops
- Custom PTFE Teflon Parts Manufacturer for Three-Necked Round Bottom Flask
- Custom PTFE Teflon Parts Manufacturer for Magnetic Stirring Bar
- Custom PTFE Teflon Parts Manufacturer Adjustable Height Flower Basket
People Also Ask
- Does graphite have a melting point? Unlocking the Extreme Heat Resistance of Graphite
- What are the advantages of graphite furnace? Achieve High-Temperature Precision and Purity
- Why graphite is used in furnace? Achieve Superior Heat Treatment & Energy Efficiency
- Why is graphite used in furnaces? For Extreme Heat, Purity, and Efficiency
- What temperature can graphite withstand? Unlocking Its Extreme Heat Potential















