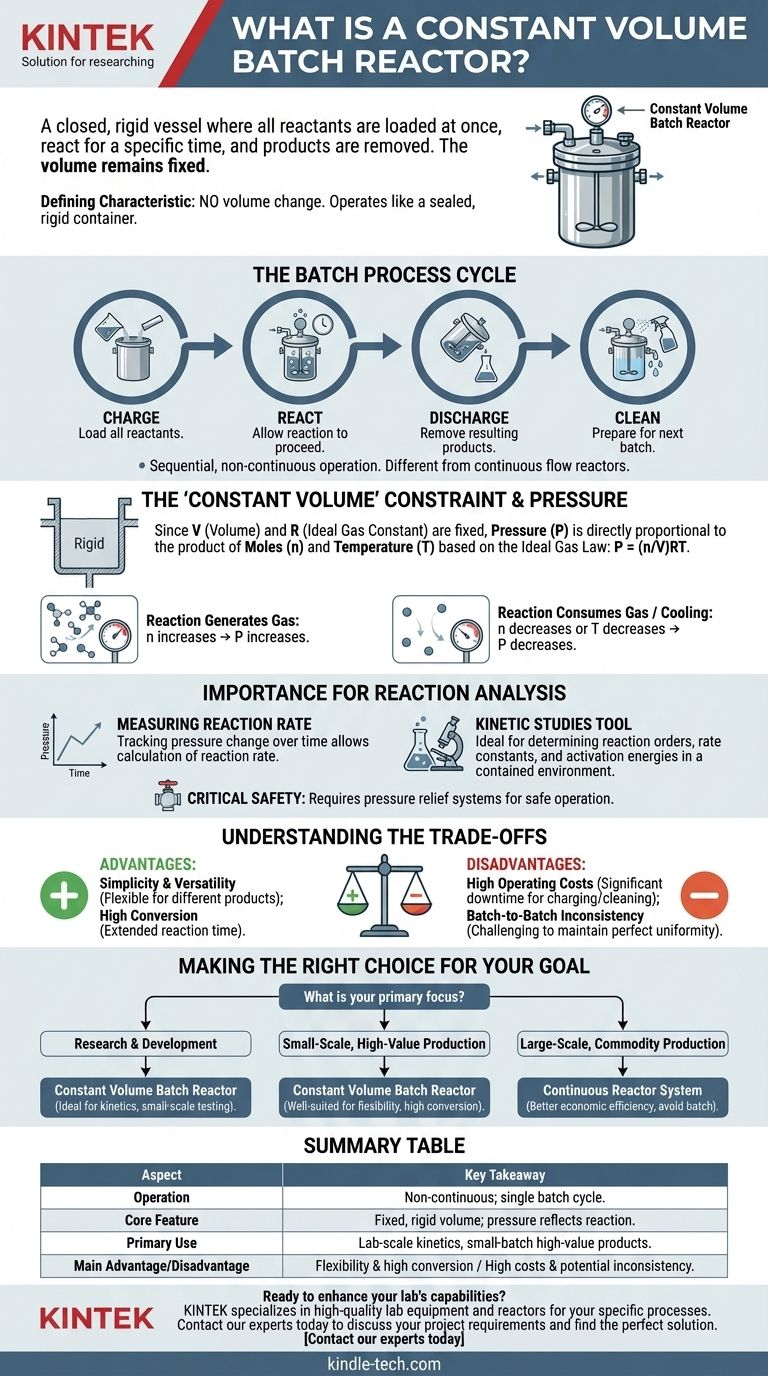
In chemical engineering, a constant volume batch reactor is a closed vessel where all reactants are loaded at once, allowed to react for a specific amount of time, and then the resulting products are removed. The defining characteristic is that the volume of the reaction space does not change during the process. This simple, non-continuous setup is a cornerstone for laboratory-scale research and for producing small quantities of high-value products.
A constant volume batch reactor operates like a sealed, rigid container. Its fixed volume means that any change in temperature or the number of gas molecules during the reaction will directly cause a change in pressure, a critical variable for both monitoring the reaction and ensuring safe operation.

The Fundamentals of Batch Reactor Operation
A batch reactor is defined by its sequential, non-continuous operation. Understanding the implications of its "constant volume" nature is key to using it effectively.
The "Batch" Process Cycle
The operation follows a distinct cycle: charge, react, discharge, and clean. All ingredients are added at the beginning, and the entire mixture is removed at the end.
This contrasts sharply with continuous reactors (like a CSTR or PFR) where reactants constantly flow in and products constantly flow out. Batch reactors are prized for their flexibility, as the same vessel can be used to make different products simply by changing the recipe for each batch.
The "Constant Volume" Constraint
The term constant volume (also known as isochoric) means the vessel is rigid and sealed. No material enters or leaves during the reaction phase.
This physical constraint has a direct and predictable effect on the system's pressure, governed by the principles of the Ideal Gas Law.
How It Connects Pressure, Moles, and Temperature
The relationship is defined by the equation P = (n/V)RT. In a constant volume reactor, V (volume) and R (the ideal gas constant) are fixed.
This creates a direct proportionality between pressure (P) and the product of the number of moles (n) and temperature (T). If a reaction generates more gas molecules, the pressure will rise. If it consumes gas molecules or if the system is cooled, the pressure will drop.
Why Constant Volume Matters for Reaction Analysis
The direct link between pressure and reaction progress is what makes constant volume batch reactors so useful, particularly in a laboratory setting.
Measuring Reaction Rate
For gas-phase reactions, tracking pressure change over time is a powerful way to measure the rate of reaction.
For example, in a reaction where one mole of gas A decomposes into two moles of gas B (A → 2B), the pressure will double if the reaction goes to completion at a constant temperature. By plotting pressure versus time, chemists can derive the kinetic parameters of the reaction.
A Tool for Kinetic Studies
Because of this measurable pressure change, constant volume batch reactors are the preferred tool for fundamental kinetic studies. They provide a clean, contained environment to determine reaction orders, rate constants, and activation energies without the complexities of flow dynamics.
Critical Safety and Design Implications
The potential for pressure change means the reactor must be engineered to withstand the maximum possible pressure generated by the reaction, including runaway scenarios.
These reactors are almost always equipped with safety features like pressure relief valves or rupture discs to prevent catastrophic failure if the pressure exceeds the vessel's design limits.
Understanding the Trade-offs
While simple, the constant volume batch reactor is not the right choice for every application. Its advantages in flexibility are offset by disadvantages in efficiency.
Advantage: Simplicity and Versatility
Batch reactors are relatively easy to design, operate, and clean. Their inherent flexibility allows a single unit to produce a wide range of different products, which is ideal for specialty chemicals, pharmaceuticals, and food production.
Advantage: High Conversion
Since reactants can be held in the reactor for an extended period, it's possible to achieve a very high conversion of reactants into products for each batch.
Disadvantage: High Operating Costs
The cycle of charging, discharging, and cleaning introduces significant downtime where no product is being made. This "non-productive" time increases labor and operational costs per unit of product, making it inefficient for large-scale manufacturing.
Disadvantage: Batch-to-Batch Inconsistency
Achieving perfect consistency from one batch to the next can be challenging. Furthermore, within a single batch, the concentration of reactants and the rate of reaction are constantly changing over time, which can sometimes lead to less uniform product quality compared to continuous systems.
Making the Right Choice for Your Goal
Selecting the right reactor type depends entirely on your scale, product, and objective.
- If your primary focus is research and development: A constant volume batch reactor is the ideal instrument for studying reaction kinetics and testing new chemical processes on a small, controlled scale.
- If your primary focus is small-scale, high-value production: This reactor is well-suited for industries like pharmaceuticals or specialty chemicals, where flexibility and high conversion are more important than massive throughput.
- If your primary focus is large-scale, low-cost commodity production: A batch reactor is almost always the wrong choice; a continuous reactor system will deliver far greater economic efficiency.
Understanding this fundamental reactor type is the first step toward designing, analyzing, and scaling chemical processes effectively.
Summary Table:
| Aspect | Key Takeaway |
|---|---|
| Operation | Non-continuous; all reactants are loaded, react, and are removed in a single batch. |
| Core Feature | Fixed, rigid volume (isochoric); pressure changes directly reflect reaction progress. |
| Primary Use | Ideal for laboratory-scale kinetic studies and small-batch, high-value product manufacturing. |
| Main Advantage | Simplicity, flexibility, and the ability to achieve high conversion per batch. |
| Main Disadvantage | High operating costs and potential for batch-to-batch inconsistency compared to continuous systems. |
Ready to enhance your lab's capabilities?
A constant volume batch reactor is fundamental for accurate R&D and small-scale production. At KINTEK, we specialize in providing high-quality lab equipment, including reactors tailored for your specific chemical processes. Our expertise ensures you get the precise tools needed for effective kinetic studies and reliable results.
Let's discuss your project requirements and find the perfect reactor solution for your laboratory.
Contact our experts today to learn more about our products and how we can support your research and production goals.
Visual Guide
Related Products
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
People Also Ask
- What is the role of a high-pressure reactor in the solvothermal synthesis of molecular sieve catalysts?
- What are the design considerations for bioreactors? Build the Perfect Environment for Cell Growth
- What are the material requirements for reaction vessels using sodium hydroxide? Simplified Lab Equipment Solutions
- What is the primary function of a sapphire glass window? Optimizing High-Throughput IR Thermography Reactors
- Why must reactors for Supercritical Water Gasification (SCWG) possess high pressure and corrosion resistance?
- What are the primary advantages of using Hastelloy C-22 for reactors? Ensure Corrosion Resistance in Hydrate Research
- What is the technical value of high-pressure reactors in HA catalyst synthesis? Optimize Mesoporous Structure Today
- What function do baffle plates serve inside a reaction vessel? Enhance Photovoltaic Backsheet Recycling Efficiency



















