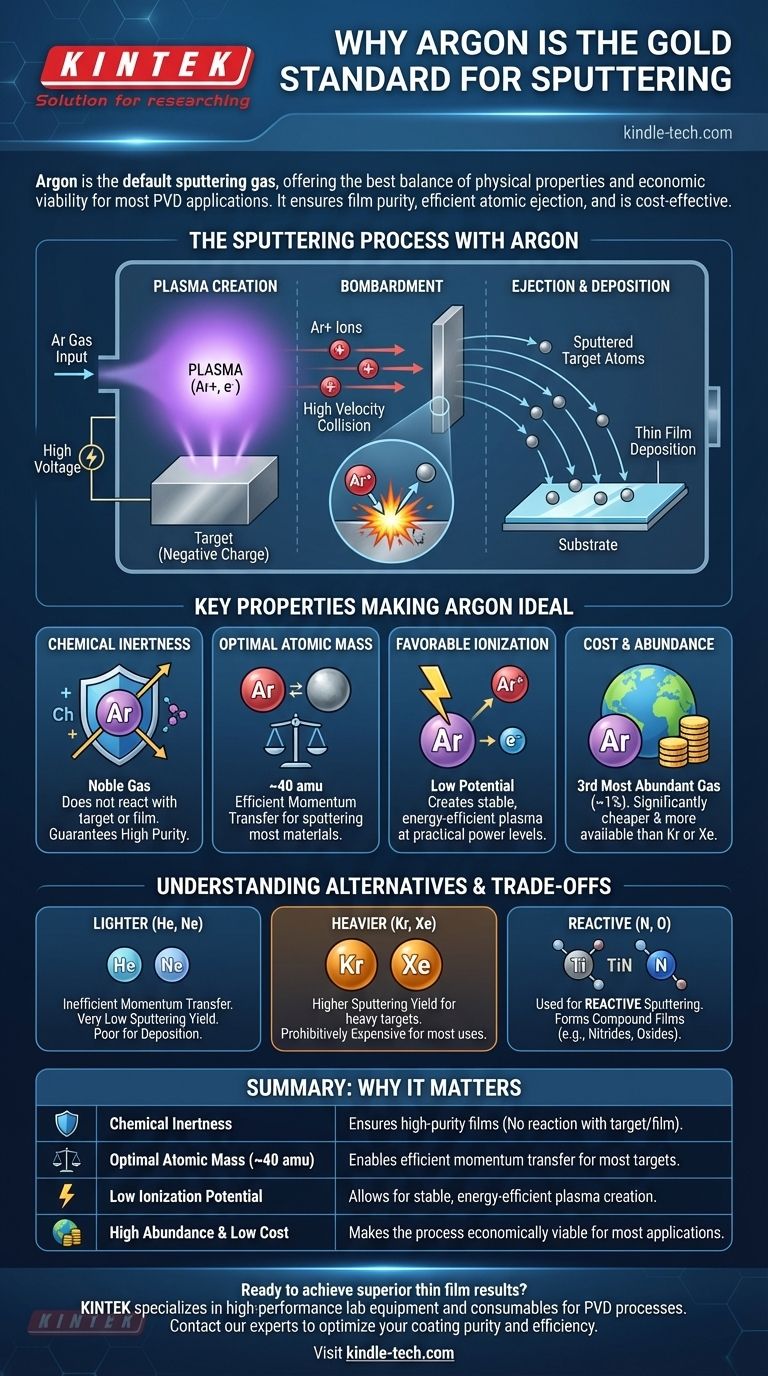
Argon is the standard gas for sputtering because it provides the best balance of physical properties and economic viability for most applications. Its chemical inertness ensures the purity of the deposited film, while its atomic mass is heavy enough to efficiently eject atoms from the target material without the high cost of even heavier noble gases.
The choice of a sputtering gas is a critical decision governed by a trade-off between sputtering efficiency, chemical reactivity, and cost. Argon's unique position as an inert, relatively heavy, and abundant noble gas makes it the default "workhorse" for the vast majority of physical vapor deposition processes.
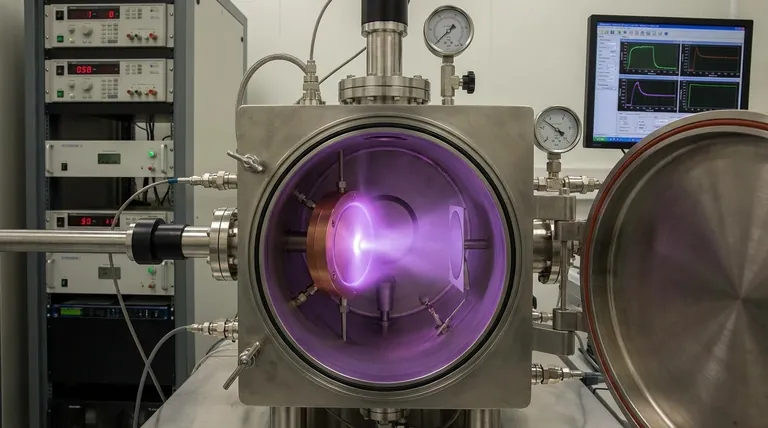
The Fundamental Role of Gas in Sputtering
To understand why argon is used, we must first understand the role of the gas itself. In sputtering, the gas is not a chemical reactant; it is a physical medium used to create ions that act like a sandblaster at the atomic level.
Creating the Plasma
The sputtering process begins by introducing a low-pressure gas, like argon, into a vacuum chamber. A high voltage is then applied, which strips electrons from the gas atoms.
This process creates a plasma, which is an energized state of matter consisting of positively charged gas ions (Ar+) and free electrons. This glowing plasma is the engine of the sputtering process.
The Bombardment Process
The material to be deposited, known as the target, is given a negative electrical charge. This attracts the positively charged argon ions from the plasma, causing them to accelerate and collide with the target surface at high velocity.
Each collision transfers kinetic energy from the argon ion to the target. If enough energy is transferred, atoms of the target material are physically ejected, or "sputtered," off the surface. These ejected atoms then travel through the chamber and deposit as a thin film on your substrate.
Key Properties That Make Argon Ideal
Argon is not the only gas that can be used for sputtering, but its specific combination of properties makes it the most effective and practical choice for depositing pure, elemental films.
1. Chemical Inertness
As a noble gas, argon is chemically inert. It will not react with the target material during bombardment or with the sputtered atoms as they travel to the substrate.
This is the most critical property for non-reactive sputtering. It guarantees that the deposited film maintains the same chemical composition as the target, ensuring high purity.
2. Optimal Atomic Mass
Effective sputtering relies on efficient momentum transfer, similar to a collision between billiard balls. The mass of the sputtering ion should be reasonably close to the mass of the target atoms.
Argon's atomic mass (around 40 amu) is heavy enough to effectively sputter most metals and other common engineering materials. Lighter gases like helium would simply bounce off, while heavier gases are often overkill and far more expensive.
3. Favorable Ionization Potential
Argon has a relatively low ionization potential, meaning it does not require an excessive amount of energy to be converted into a plasma.
This allows for the creation of a stable, dense plasma at practical power levels, making the entire process more energy-efficient compared to gases that are harder to ionize.
4. Cost and Abundance
From a practical standpoint, argon's greatest advantage is its availability. It is the third most abundant gas in Earth's atmosphere (at ~1%).
This abundance makes it significantly cheaper and more readily available than other suitable noble gases like Krypton or Xenon, which are much rarer and therefore orders of magnitude more expensive.
Understanding the Alternatives and Their Trade-offs
Choosing a different gas fundamentally changes the sputtering process, introducing specific benefits and significant drawbacks.
Lighter Gases (Helium, Neon)
Helium and Neon are poor choices for deposition. Due to their low atomic mass, momentum transfer is highly inefficient, resulting in a very low sputtering yield (the number of target atoms ejected per incident ion). Their primary use is in analytical techniques or for very gentle substrate cleaning, not for building a film.
Heavier Gases (Krypton, Xenon)
Krypton and Xenon are significantly heavier than argon and can produce a higher sputtering yield for very heavy target materials. This can increase deposition rates.
However, their extreme rarity makes them prohibitively expensive for all but the most specialized, high-value industrial or research applications where maximizing the sputtering rate is the absolute primary concern.
Reactive Gases (Nitrogen, Oxygen)
Gases like nitrogen and oxygen are used in a process called reactive sputtering. Here, the gas is intentionally chosen to react with the sputtered target atoms.
For example, by sputtering a titanium target in a nitrogen/argon atmosphere, you don't deposit a pure titanium film. Instead, you form a hard, gold-colored Titanium Nitride (TiN) ceramic film on the substrate. This is how many hard coatings and optical films are made.
Making the Right Choice for Your Application
Selecting the correct gas is essential for controlling the outcome of your deposition process.
- If your primary focus is cost-effective deposition of pure metals or materials: Argon is the undisputed standard choice due to its ideal balance of performance, purity, and cost.
- If your primary focus is forming a compound film like a nitride or an oxide: You must use a reactive gas like nitrogen or oxygen, typically mixed with argon to stabilize the plasma.
- If your primary focus is maximizing deposition rate for a niche, high-value process: Heavier noble gases like Krypton or Xenon can be considered, but only if the significant cost increase is justifiable.
Ultimately, understanding the role of the sputtering gas gives you precise control over the properties of your resulting thin film.
Summary Table:
| Property | Why It Matters for Sputtering |
|---|---|
| Chemical Inertness | Ensures high-purity films by not reacting with the target or deposited material. |
| Optimal Atomic Mass (~40 amu) | Enables efficient momentum transfer to eject atoms from most target materials. |
| Low Ionization Potential | Allows for stable plasma creation at practical, energy-efficient power levels. |
| High Abundance & Low Cost | Makes the process economically viable for most industrial and research applications. |
Ready to achieve superior thin film results in your lab? The right sputtering gas is just one part of the equation. KINTEK specializes in high-performance lab equipment and consumables for all your deposition needs. Contact our experts today to optimize your PVD process and ensure the purity and efficiency of your coatings.
Visual Guide
Related Products
- Evaporation Boat for Organic Matter
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Aluminized Ceramic Evaporation Boat for Thin Film Deposition
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Lab Sterile Slapping Type Homogenizer for Tissue Mashing and Dispersing
People Also Ask
- Why is an ultrasonic cleaner used with ethanol to treat alloy specimens? Ensure Superior Diffusion Bonding Results
- What is the disadvantage of plate frame filter press? High Labor Costs and Batch Process Downtime
- Why is industrial ultrasonic cleaning equipment necessary for UNS S32750 preparation? Ensure Plasma Nitriding Success
- What is the difference between cast and sintered parts? Choose the Right Metal Forming Process
- What is the oven used in microbiology lab? A Guide to Hot Air Ovens for Sterilization
- What is the energy consumption of conventional ultra-low temperature (ULT) freezers? Managing High Energy Costs
- What is the carbon footprint of diamond mining? Uncovering the True Environmental and Ethical Cost
- What are the limitations of sputtering process? Understand Key Trade-offs for Thin Film Deposition

















