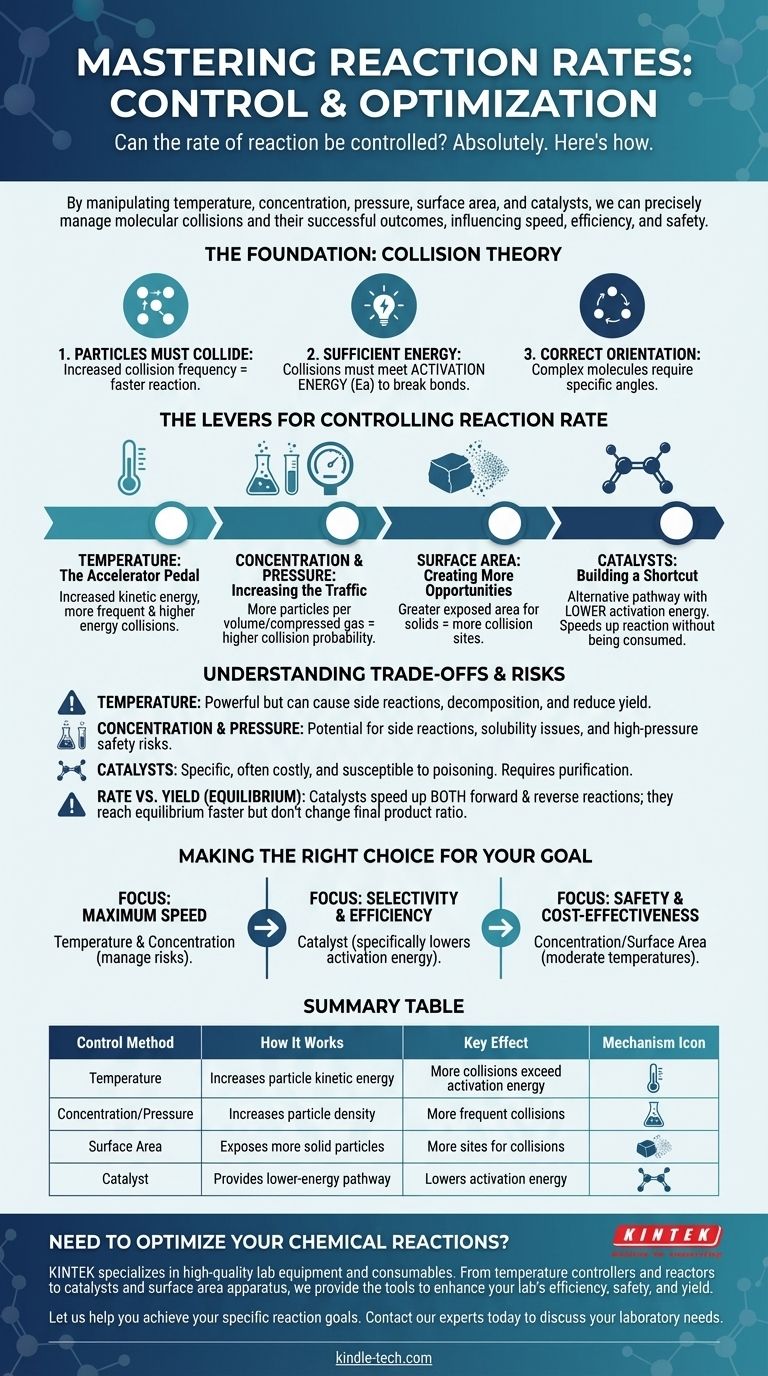
Absolutely. The rate of a chemical reaction is not a fixed property; it can be precisely controlled. By manipulating specific physical and chemical factors, we can deliberately speed up, slow down, or even halt a reaction. The primary levers for this control are temperature, concentration of reactants, pressure (for gases), surface area (for solids), and the introduction of catalysts.
Controlling a reaction's rate is fundamentally about managing the frequency and energy of molecular collisions. Every method, from heating a substance to adding a catalyst, works by influencing how often particles collide and whether those collisions are successful.

The Foundation: Collision Theory
To control a reaction, you must first understand what makes it happen. The rate of any reaction is governed by the principles of collision theory, which states that three conditions must be met for a reaction to occur.
1. Particles Must Collide
For two or more molecules to react, they must first come into physical contact. The more frequently particles collide, the more opportunities they have to react, which increases the reaction rate.
2. Collisions Must Have Sufficient Energy
Simply colliding is not enough. The colliding particles must possess a minimum amount of combined kinetic energy, known as the activation energy (Ea). This energy is required to break existing chemical bonds so that new ones can form.
Collisions with less than the activation energy will be unsuccessful; the particles will simply bounce off each other unchanged.
3. Particles Must Have the Correct Orientation
For complex molecules, the collision must also happen at a specific angle or orientation. If the reactive parts of the molecules do not align properly during the collision, no reaction will occur, even if the energy requirement is met.
The Levers for Controlling Reaction Rate
Understanding collision theory gives us a clear roadmap for manipulating reaction speed. Each control method works by altering one or more of the conditions required for a successful collision.
Temperature: The Accelerator Pedal
Increasing the temperature raises the average kinetic energy of the particles. This has a powerful, twofold effect.
First, it causes particles to move faster, leading to more frequent collisions. Second, and more importantly, it means a much larger fraction of those collisions will have energy equal to or greater than the activation energy, dramatically increasing the rate of successful reactions.
Concentration and Pressure: Increasing the Traffic
Increasing the concentration of reactants in a solution means there are more particles packed into the same volume. This directly increases the probability of collision, thus raising the reaction rate.
For gases, increasing the pressure has the same effect. It forces gas molecules closer together, effectively increasing their concentration and leading to more frequent collisions.
Surface Area: Creating More Opportunities
This factor is critical when a solid reacts with a liquid or gas. By breaking a solid into smaller pieces (e.g., from a solid chunk to a powder), you dramatically increase its total surface area.
This exposes more particles of the solid to the other reactant, creating far more sites where collisions can occur and increasing the overall reaction rate.
Catalysts: Building a Shortcut
A catalyst is a substance that increases reaction rate without being consumed in the process. It works by providing an alternative reaction pathway with a lower activation energy.
A catalyst doesn't make particles collide more often or with more energy. Instead, it lowers the energy "barrier" a collision must overcome to be successful, making it much easier for a reaction to proceed.
Understanding the Trade-offs and Risks
While these levers are effective, they are not without consequences. Choosing the right method requires understanding their limitations and potential downsides.
The Blunt Force of Temperature
Heat is a powerful but indiscriminate tool. While it will speed up your desired reaction, it will also speed up any potential side reactions. At very high temperatures, it can even cause reactants or products to decompose, lowering your overall yield.
The Dangers of Concentration and Pressure
High concentrations can sometimes lead to unwanted side reactions or solubility issues. More critically, operating at very high pressures requires specialized, expensive, and robust equipment to manage the significant safety risks of a potential containment failure.
The Specificity and Cost of Catalysts
Catalysts are often highly specific and can be very expensive (e.g., those using platinum or palladium). They can also be rendered useless by impurities, a process known as catalyst poisoning, which requires careful purification of reactants.
Rate vs. Yield (Equilibrium)
It is crucial to distinguish between reaction rate and reaction yield. For reversible reactions, which can proceed in both forward and reverse directions, a catalyst speeds up both reactions equally. This means you will reach equilibrium faster, but it will not change the final ratio of products to reactants.
Making the Right Choice for Your Goal
The optimal strategy for controlling a reaction depends entirely on your specific objective, whether it's speed, efficiency, or safety.
- If your primary focus is maximum speed: Increasing temperature and concentration are your most direct tools, but you must actively manage the risks of side reactions and safety hazards.
- If your primary focus is selectivity and efficiency: A catalyst is often the best choice, as it can specifically lower the activation energy for your desired reaction without promoting others.
- If your primary focus is safety and cost-effectiveness: Manipulating concentration or surface area at moderate temperatures are often the most accessible and lowest-risk methods.
Mastering these factors allows you to transform chemical reactions from fixed events into dynamic processes that can be precisely directed to achieve a specific outcome.
Summary Table:
| Control Method | How It Works | Key Effect |
|---|---|---|
| Temperature | Increases particle kinetic energy | More collisions exceed activation energy |
| Concentration/Pressure | Increases particle density | More frequent collisions |
| Surface Area | Exposes more solid reactant particles | More sites for collisions |
| Catalyst | Provides a lower-energy reaction pathway | Lowers activation energy |
Need to Optimize Your Chemical Reactions?
Precisely controlling reaction rates is key to successful lab work. Whether you need to accelerate a synthesis, improve selectivity, or ensure safe operation, the right equipment is essential.
KINTEK specializes in providing high-quality lab equipment and consumables to help you master these variables. From precision temperature controllers and reactors for managing heat and pressure to a wide range of catalysts and apparatus designed for optimal surface area interaction, we have the tools to enhance your lab's efficiency, safety, and yield.
Let us help you achieve your specific reaction goals. Contact our experts today to discuss your laboratory needs and find the perfect solution.
Visual Guide
Related Products
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Customizable Laboratory High Temperature High Pressure Reactors for Diverse Scientific Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Warm Isostatic Press WIP Workstation 300Mpa for High Pressure Applications
People Also Ask
- What is the contribution of a hydrothermal reactor to graded pore construction? Precision Templates for TAS
- What is the function of a constant temperature hydrothermal reactor? Master Coal Fly Ash Activation
- What is the purpose of using high-purity argon gas in a high-pressure reactor? Ensure Precise Corrosion Test Data
- What is the role of high-pressure reactors in the study of alloy oxidation? Essential Tools for Supercritical Research
- What role does an autoclave play in simulating PWR conditions? Advanced Material Validation for Nuclear Safety



















