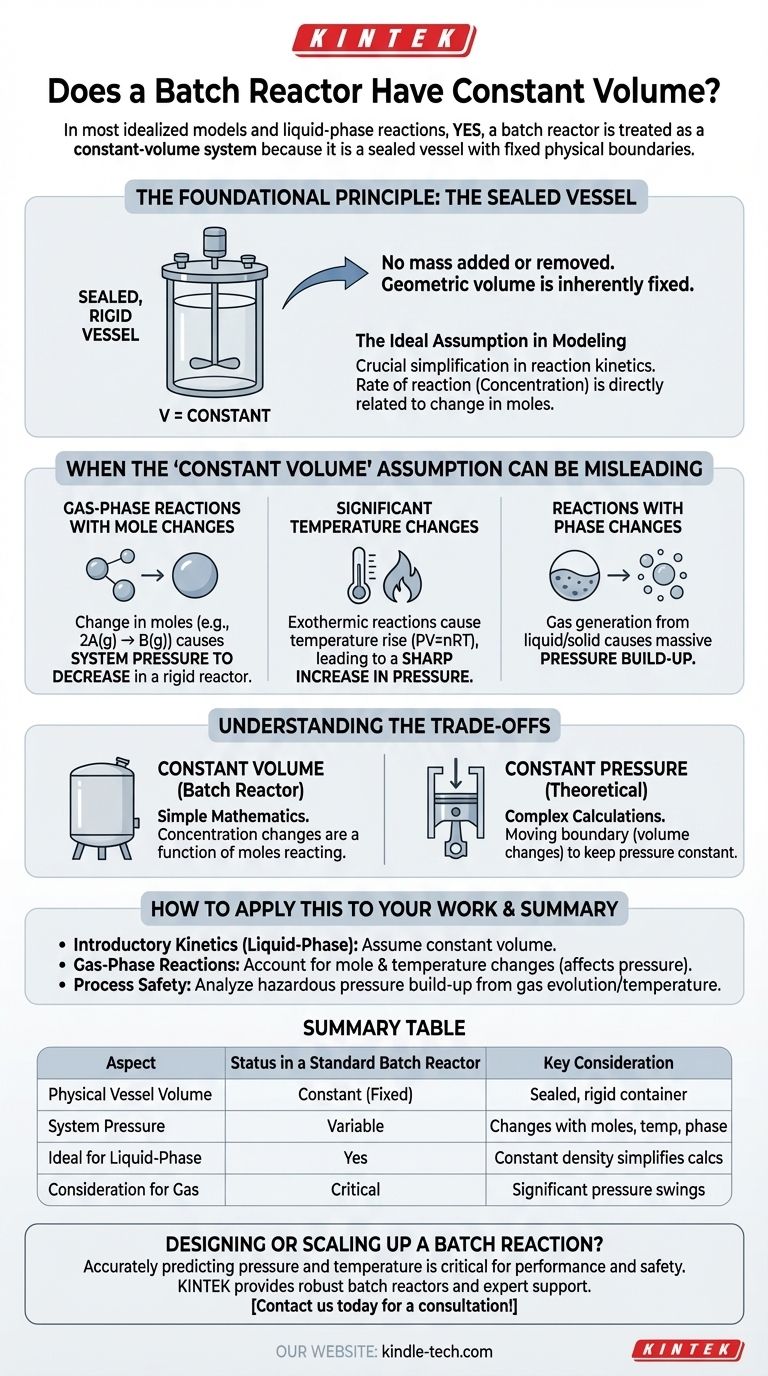
In most idealized models and for liquid-phase reactions, yes, a batch reactor is treated as a constant-volume system. This is because a batch reactor is fundamentally a sealed vessel with fixed physical boundaries, meaning the total volume of the container itself does not change during the reaction.
The critical distinction is not whether the vessel's volume is constant, but whether the reacting medium within it maintains a constant density. While the physical reactor volume is fixed, changes in moles, temperature, or phase can alter system pressure and invalidate the simplifying assumption of constant volume for kinetic calculations.

The Foundational Principle: The Sealed Vessel
The assumption of constant volume stems directly from the physical design and definition of a batch reactor. It is a closed system where no mass is added or removed during the reaction.
The Physical Definition
A batch reactor is a container, often with an agitator, that is loaded with reactants, sealed, and allowed to react for a specified time. Because it is a sealed, rigid vessel, its geometric volume is inherently fixed.
The Ideal Assumption in Modeling
For chemical engineers and chemists, this physical reality allows for a crucial simplification in reaction kinetics. The rate of reaction is often expressed in terms of concentration (e.g., moles per liter). If the volume (V) is constant, the change in concentration is directly and simply related to the change in the number of moles.
When the "Constant Volume" Assumption Can Be Misleading
While the vessel's volume is fixed, certain reaction conditions can make the assumption of constant volume problematic for calculations, particularly in gas-phase systems.
Gas-Phase Reactions with Mole Changes
Consider a gas-phase reaction where the number of moles changes, such as 2A(g) -> B(g). In a rigid, constant-volume reactor, the number of gas molecules is halved, which would cause the system pressure to decrease significantly (assuming constant temperature). The volume of the vessel hasn't changed, but the properties of the reacting fluid have.
Significant Temperature Changes
The ideal gas law (PV = nRT) shows that pressure, volume, and temperature are all related. If a gas-phase reaction is highly exothermic, the temperature will rise dramatically. In a fixed-volume reactor, this will cause a sharp increase in pressure. While volume is constant, the state of the system is changing in other ways that must be accounted for.
Reactions with Phase Changes
A reaction that produces a gas from a liquid or solid (e.g., decomposition of calcium carbonate) will cause a massive build-up of pressure inside the fixed-volume reactor. The volume of the reacting mixture has effectively expanded, even though the vessel volume is constant.
Understanding the Trade-offs: Constant Volume vs. Constant Pressure
The distinction becomes clearest when comparing a constant volume batch reactor to a theoretical constant pressure system.
The Simplicity of Constant Volume
Most lab and industrial batch reactors are rigid tanks. Assuming constant volume makes the mathematics of reaction rate equations far more straightforward, as concentration changes are only a function of the moles reacting.
The Complexity of Constant Pressure
A constant pressure system would require a moving boundary, like a piston in a cylinder. As a reaction changes the number of gas moles or temperature, the piston would move to keep pressure constant, meaning the volume would actively change. This requires more complex calculations where both concentration and volume are variables.
How to Apply This to Your Work
Your approach depends entirely on the problem you are trying to solve.
- If your primary focus is introductory chemical kinetics: Assume the batch reactor has a constant volume, especially for liquid-phase reactions, as this is the standard for simplifying rate equations.
- If your primary focus is designing gas-phase reactions: You must account for changes in the number of moles and temperature, as this will directly impact system pressure in a constant-volume reactor.
- If your primary focus is process safety: Never assume constant volume is the same as constant pressure; rigorously analyze how gas evolution or temperature changes can create hazardous pressure build-up.
Ultimately, understanding the difference between the reactor's fixed physical volume and the dynamic behavior of the reacting medium is the key to accurate and safe chemical engineering.
Summary Table:
| Aspect | Status in a Standard Batch Reactor | Key Consideration |
|---|---|---|
| Physical Vessel Volume | Constant (Fixed) | The reactor is a sealed, rigid container. |
| System Pressure | Variable | Changes with mole count, temperature, and phase. |
| Ideal for Liquid-Phase Kinetics | Yes | Constant density simplifies concentration calculations. |
| Consideration for Gas-Phase Reactions | Critical | Mole changes cause significant pressure swings. |
Designing or scaling up a batch reaction? Accurately predicting pressure and temperature changes is critical for both performance and safety. KINTEK specializes in lab equipment and consumables, providing robust batch reactors and expert support for your laboratory needs. Let our experts help you select the right reactor for your process—contact us today for a consultation!
Visual Guide
Related Products
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Super Sealed Electrolytic Electrochemical Cell
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Manual Cold Isostatic Pressing Machine CIP Pellet Press
People Also Ask
- Why is glass lined reactor blue? The Science Behind Cobalt Oxide for Durability
- What is the function of a reactor with a high-speed paddle stirrer? Master Precise Magnesium Hydroxide Precipitation
- What is the function of a constant temperature hydrothermal reactor? Master Coal Fly Ash Activation
- Why is a high-pressure batch catalytic reactor necessary for ADN? Elevate Your Propellant Characterization
- What is the function of high-temperature and high-pressure reactors in SCWO? Explore Material Science Insights
- How do a high-pressure reactor and a high-precision injection pump collaborate in experiments? Optimize CO2 Storage
- How do high-pressure reactors contribute to corrosion resistance evaluation? Simulating Deep Geological Disposal
- What experimental conditions do high-pressure reactors provide for studying the chemical corrosion of epoxy resins?



















