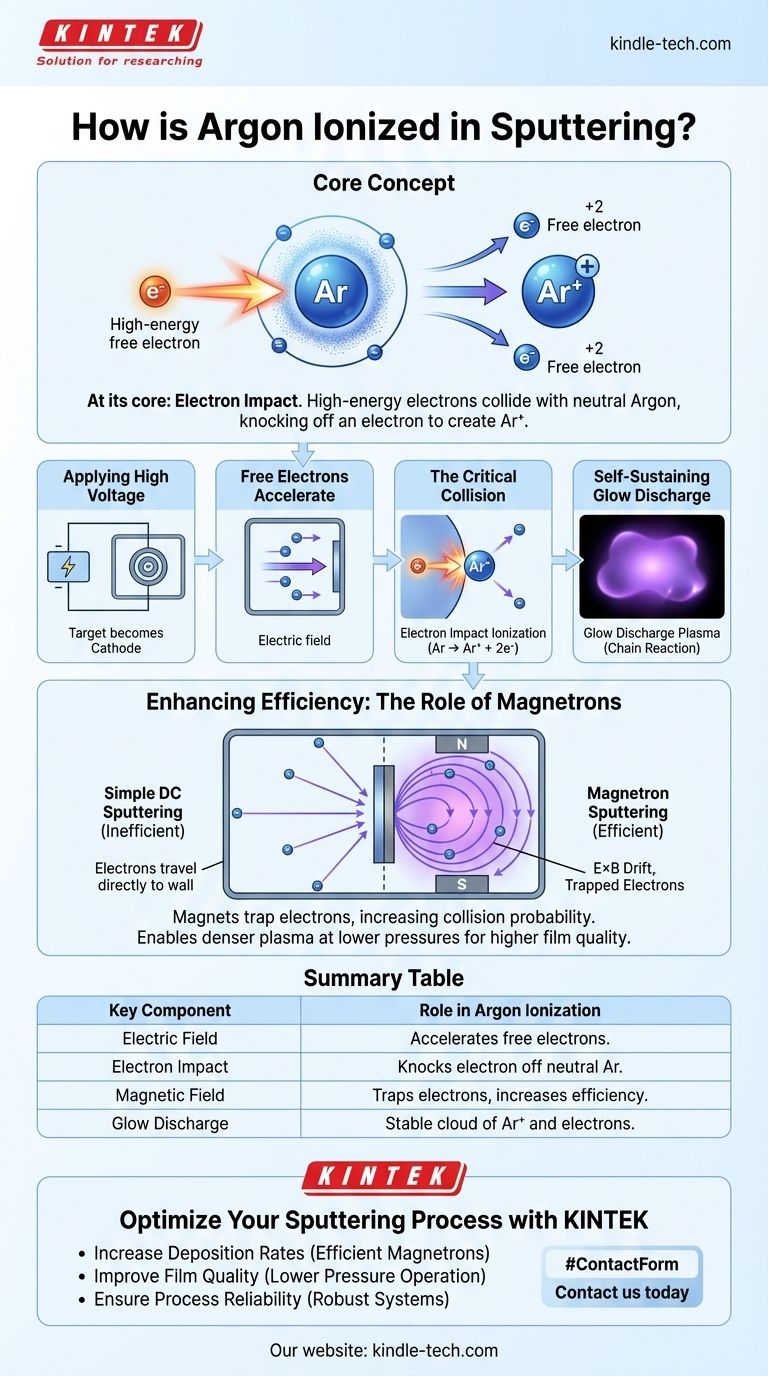
At its core, argon is ionized in sputtering through electron impact. A strong electric field accelerates free electrons to high energies within the vacuum chamber. When one of these energetic electrons collides with a neutral argon atom, it knocks another electron loose, creating a positively charged argon ion (Ar+) and a second free electron, which continues the process.
The essential goal is not merely to ionize a few atoms, but to ignite and sustain a stable plasma. This is achieved by applying a high voltage to create the initial electron-atom collisions, and in modern systems, by using magnetic fields to trap electrons, dramatically increasing the efficiency of this ionization cascade.

The Fundamental Process: Creating a Plasma
To understand sputtering, you must first understand how the inert gas, typically argon, is converted into an active plasma. This process relies on a few key steps.
The Initial Spark: Applying High Voltage
The process begins by placing the material to be sputtered (the target) into a vacuum chamber and applying a strong negative DC or RF voltage to it. This makes the target a cathode.
The Role of Free Electrons
Within any low-pressure gas, there are always a small number of stray, free electrons. The powerful electric field created by the voltage immediately accelerates these negatively charged electrons away from the negative target at very high speeds.
The Critical Collision
As these high-energy electrons travel through the chamber, they inevitably collide with the much larger, neutral argon atoms. If an electron has enough energy, it will strip an electron from the argon atom's outer shell.
This event, called electron impact ionization, is the crucial step. The result is one positively charged argon ion (Ar+) and two free electrons.
A Self-Sustaining Glow Discharge
This process creates a chain reaction. The original electron and the newly freed electron are both accelerated by the electric field, going on to strike and ionize more argon atoms. This cascade quickly creates a stable, visible cloud of ions and electrons known as a glow discharge plasma.
Enhancing Efficiency: The Role of Magnetrons
Simple DC sputtering is functional but inefficient. Many electrons travel from the cathode directly to the chamber walls (the anode) without ever striking an argon atom, requiring higher gas pressures to ensure enough collisions occur. Magnetron sputtering solves this problem.
How Magnets Trap Electrons
In a magnetron system, powerful magnets are placed behind the target. This creates a magnetic field that is perpendicular to the electric field near the target's surface.
This combination of fields forces the electrons into a long, spiral path, effectively trapping them in a zone directly in front of the target. This is known as the E×B drift.
The Benefit of a Longer Path
By forcing electrons to travel a much greater distance before eventually escaping, their probability of colliding with and ionizing an argon atom increases by orders of magnitude.
Why This Matters for Sputtering
This immense increase in ionization efficiency is the primary advantage of magnetron sputtering. It allows a dense, stable plasma to be formed at much lower pressures. Lower pressure means fewer sputtered atoms will collide with gas on their way to the substrate, preserving their energy and resulting in a denser, higher-quality film.
Common Pitfalls and Key Parameters
Achieving a stable and effective plasma requires balancing several variables. Understanding their interplay is key to process control.
Pressure vs. Mean Free Path
The pressure of the argon gas determines the "mean free path"—the average distance a particle travels before a collision.
- Too low: Not enough argon atoms are present, leading to an unstable plasma.
- Too high: Sputtered atoms lose too much energy in gas collisions, reducing deposition rate and film quality.
Voltage and Power
The applied voltage dictates the energy of the electrons and ions. Higher voltage leads to more energetic ion bombardment on the target, which typically increases the sputter yield (the number of atoms ejected per incoming ion).
A Critical Misconception
It is a common error to think that the magnets directly ionize the argon. The magnets do not ionize anything. Their sole function is to confine the electrons that perform the ionization, making the process radically more efficient.
Making the Right Choice for Your Goal
The method and parameters you use for ionization directly impact the final result of your deposition.
- If your primary focus is a basic understanding: Remember that ionization is simply a high-energy electron knocking another electron off a neutral argon atom.
- If your primary focus is process efficiency: The key is using magnets to trap electrons near the target, which creates a denser plasma at lower pressures and increases deposition rates.
- If your primary focus is film quality: Efficient ionization via magnetrons is critical, as it allows for lower operating pressures, which reduces gas impurities in your final film and improves its density.
Ultimately, mastering the ionization process is the first step toward controlling the quality and efficiency of any sputtering deposition.
Summary Table:
| Key Component | Role in Argon Ionization |
|---|---|
| Electric Field | Accelerates free electrons to high energies for collisions. |
| Electron Impact | High-energy electron knocks an electron off a neutral argon atom (Ar → Ar⁺). |
| Magnetic Field (Magnetron) | Traps electrons, increasing their path length and ionization efficiency. |
| Glow Discharge Plasma | The resulting stable cloud of argon ions (Ar⁺) and electrons. |
Optimize Your Sputtering Process with KINTEK
Achieving a stable, efficient plasma is fundamental to high-quality thin film deposition. Whether you are developing new coatings or optimizing an existing process, the right lab equipment is critical.
KINTEK specializes in advanced sputtering systems and consumables for all your laboratory needs. Our expertise can help you:
- Increase Deposition Rates with efficient magnetron sources.
- Improve Film Quality by enabling lower-pressure operation.
- Ensure Process Reliability with robust equipment and support.
Contact us today to discuss how our solutions can enhance your research and production. Let's ignite your next breakthrough together.
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- VHP Sterilization Equipment Hydrogen Peroxide H2O2 Space Sterilizer
- 304 316 Stainless Steel Vacuum Ball Valve Stop Valve for High Vacuum Systems
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
People Also Ask
- What are the applications of PECVD? Essential for Semiconductors, MEMS, and Solar Cells
- What are the drawbacks of PECVD? Understanding the Trade-offs of Low-Temperature Deposition
- What are the advantages of PECVD? Enable Low-Temperature, High-Quality Thin-Film Deposition
- What is the plasma CVD process? Achieve Low-Temperature Thin Film Deposition
- Why does PECVD commonly use RF power input? For Precise Low-Temperature Thin Film Deposition



















