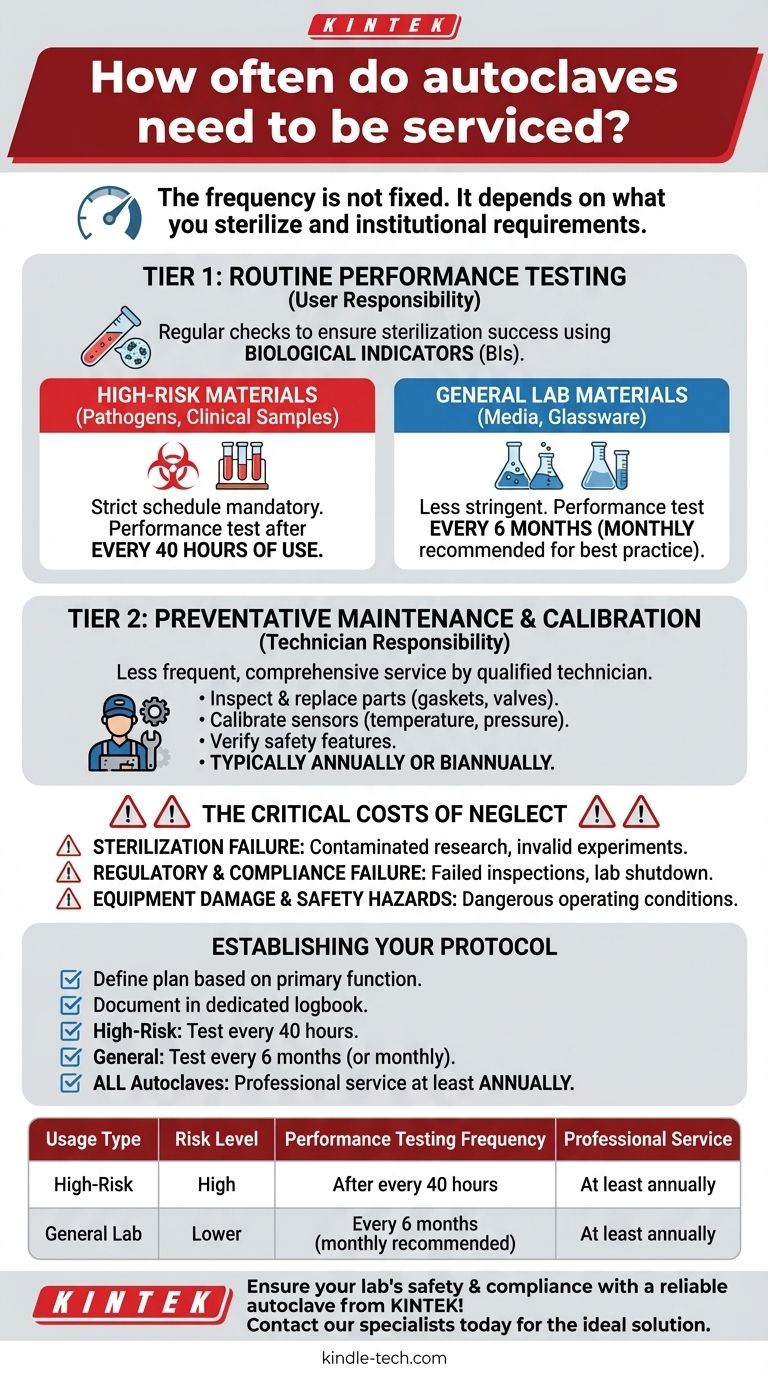
The frequency of autoclave servicing is not a single, fixed interval. It is determined entirely by what you are sterilizing and the specific requirements of your institution. For high-risk materials like human pathogens or clinical samples, performance testing is required after every 40 hours of use, while autoclaves for general materials may only require testing every six months.
An autoclave's maintenance schedule is a layered system, not a single event. It combines frequent, user-performed performance tests with less frequent professional servicing, with the exact frequency dictated by the biohazardous risk of the materials being processed.

Understanding the Two Tiers of Autoclave Maintenance
"Servicing" is often used as a catch-all term, but it's crucial to distinguish between routine performance validation and deeper preventative maintenance.
Tier 1: Routine Performance Testing (User Responsibility)
This is the most frequent requirement and serves as a regular check to ensure the autoclave is achieving successful sterilization in every cycle.
This testing is typically done using biological indicators (BIs), which contain highly resistant spores. If the autoclave successfully kills these spores, you can be confident that it is effectively sterilizing your load.
Tier 2: Preventative Maintenance & Calibration (Technician Responsibility)
This is a less frequent but more comprehensive service performed by a qualified technician, often annually or biannually.
This service includes inspecting and replacing wear-and-tear parts like door gaskets and valves, calibrating temperature and pressure sensors, and verifying safety features. This is critical for the long-term reliability and safety of the unit.
How Usage Dictates Your Testing Schedule
The risk associated with sterilization failure is the primary factor determining your required testing frequency. Always defer to your institution's specific written policy, which may be stricter than these general guidelines.
For High-Risk Materials (Pathogens, Human Samples)
If your autoclave is used to inactivate human pathogens, blood, tissues, or other potentially infectious clinical samples, a strict testing schedule is mandatory.
The standard requirement is to perform a performance test with a biological indicator after every 40 hours of use. This high frequency ensures that any failure is caught immediately, preventing the release of hazardous material or the use of non-sterile equipment in critical applications.
For General Laboratory Materials (Media, Glassware)
For autoclaves used to sterilize non-infectious materials like culture media, glassware, or instruments for general research, the requirements are less stringent.
In these cases, performance testing is typically required every six months. However, many institutions adopt a more conservative internal policy, such as testing at least once per month, to ensure a consistent state of validation.
The Critical Costs of Neglect
Failing to adhere to a proper testing and maintenance schedule introduces significant risks that go far beyond a single failed cycle.
Risk 1: Sterilization Failure
This is the most direct consequence. It can lead to contaminated research, failed experiments, and the potential use of non-sterile equipment in sensitive procedures, invalidating weeks or months of work.
Risk 2: Regulatory & Compliance Failure
Autoclave logs are one of the first things auditors and safety inspectors check. A lack of complete and consistent testing records is a major red flag that can lead to a failed inspection and potential shutdown of lab operations.
Risk 3: Equipment Damage and Safety Hazards
An uncalibrated or poorly maintained autoclave can become a safety hazard. Malfunctioning safety valves or inaccurate pressure sensors can create dangerous operating conditions in a high-pressure, high-temperature vessel.
Establishing Your Autoclave Maintenance Protocol
Use your autoclave's primary function to define your maintenance plan. Document everything in a dedicated logbook kept with the machine.
- If your primary focus is inactivating clinical or biohazardous waste: You must perform and document a biological indicator test after every 40 hours of operation.
- If your primary focus is sterilizing general lab media and glassware: You must test at least every six months, but adopt a monthly testing schedule for best practice and to exceed compliance standards.
- If you are responsible for any autoclave: You must ensure it is professionally serviced and calibrated at least once a year, or per the manufacturer's recommendation, in addition to your routine performance tests.
A rigorously maintained autoclave is the foundation of safe, repeatable, and compliant scientific work.
Summary Table:
| Usage Type | Risk Level | Performance Testing Frequency | Professional Service |
|---|---|---|---|
| High-Risk (Pathogens, Clinical Samples) | High | After every 40 hours of use | At least annually |
| General Lab Materials (Media, Glassware) | Lower | Every 6 months (monthly recommended) | At least annually |
Ensure your lab's safety and compliance with a reliable autoclave from KINTEK!
Our high-performance autoclaves are built for precision and durability, perfectly suited for handling everything from high-risk biohazardous materials to everyday sterilization needs. Backed by expert technical support and comprehensive service plans, we help you maintain peak performance and meet strict regulatory standards.
Don't leave your sterilization to chance—contact our specialists today to find the ideal autoclave solution for your laboratory!
Visual Guide
Related Products
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Test Sieves and Sieving Machines
- Benchtop Laboratory Vacuum Freeze Dryer
People Also Ask
- Why is zirconium preferred as a lining material for HPAL autoclaves? Ensure Unmatched Corrosion Resistance
- How often should a dental autoclave be cleaned? A Daily, Weekly, and Monthly Guide
- What is the purpose of treating polyester fabric in an autoclave? Ensure Reliable Results in Lab Experiments
- How do batch high-pressure autoclaves facilitate the catalytic hydrogenation of glucose? Boost Sorbitol Yield to 99%+
- What is the benefit of autoclave? Achieve Rapid, Reliable Sterilization for Your Lab
- What is a lab autoclave? Your Guide to Sterilization with Pressurized Steam
- How do you sterilize medical equipment in an autoclave? A Guide to a Fail-Safe Process
- What is a laboratory autoclave? A Guide to Sterilization with Pressurized Steam



















