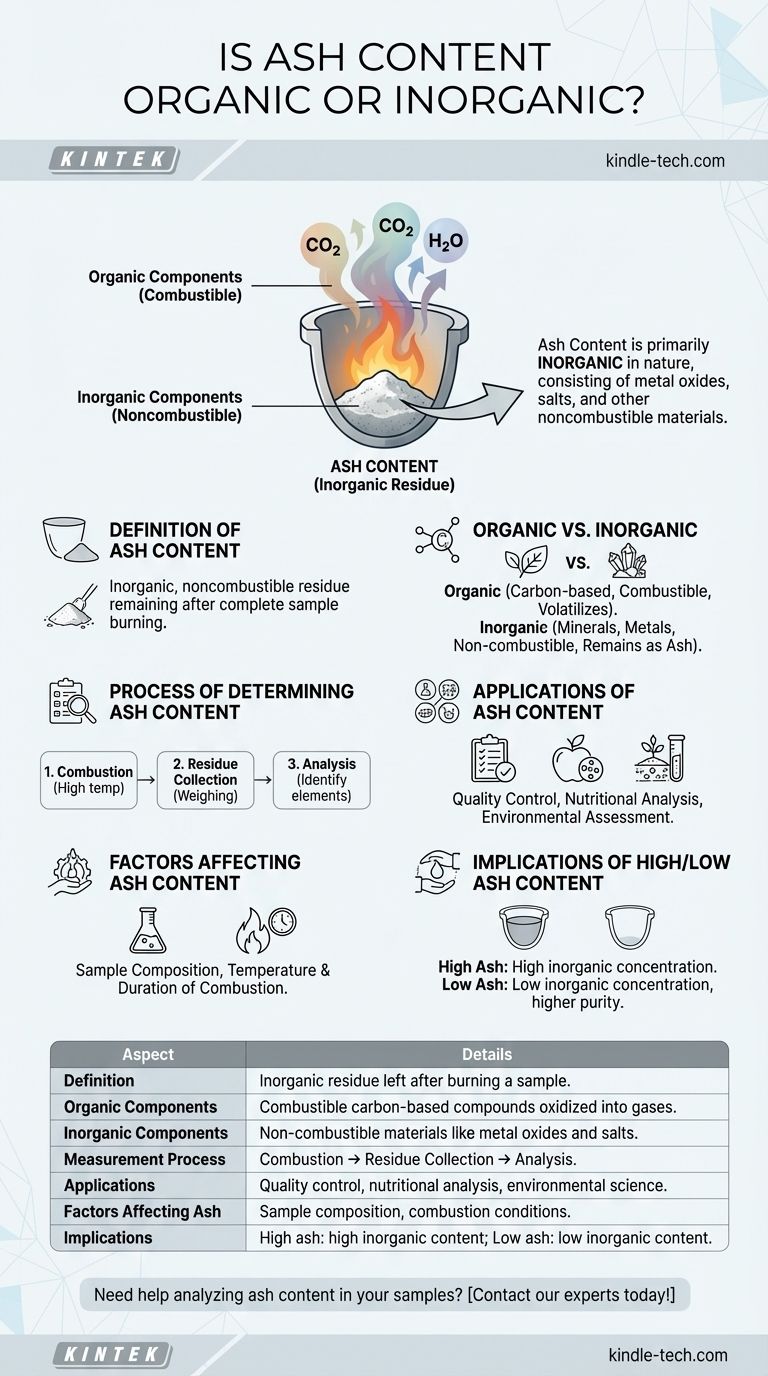
Ash content is primarily inorganic in nature. When a sample is completely burned, the organic components are oxidized and volatilized, leaving behind inorganic residues such as metal oxides, salts, and other noncombustible materials. These residues are what constitute the ash content. The process of burning effectively separates the organic matter from the inorganic components, making ash content a reliable measure of the inorganic material in a sample.

Key Points Explained:
-
Definition of Ash Content:
- Ash content refers to the inorganic, noncombustible residue that remains after a sample is completely burned. This residue typically consists of metal oxides, salts, and other inorganic compounds.
-
Organic vs. Inorganic Components:
- Organic Components: These are primarily carbon-based compounds that are combustible. When burned, they are oxidized into gases like carbon dioxide and water vapor, leaving little to no solid residue.
- Inorganic Components: These include minerals, metals, and other non-carbon-based compounds that do not combust. They remain as solid residues after the burning process.
-
Process of Determining Ash Content:
- Combustion: The sample is heated to high temperatures until all organic matter is burned off.
- Residue Collection: The remaining inorganic material is collected and weighed to determine the ash content.
- Analysis: The composition of the ash can be further analyzed to identify specific inorganic elements present.
-
Applications of Ash Content Measurement:
- Quality Control: In industries like food, pharmaceuticals, and agriculture, ash content is used to assess the purity and quality of products.
- Nutritional Analysis: In food science, ash content can indicate the mineral content of food products.
- Environmental Science: Ash content is used to analyze soil and sediment samples to determine their inorganic composition.
-
Factors Affecting Ash Content:
- Sample Composition: The type and amount of inorganic materials in the original sample will directly affect the ash content.
- Combustion Conditions: Temperature and duration of combustion can influence the completeness of organic matter removal and the stability of inorganic residues.
-
Implications of High or Low Ash Content:
- High Ash Content: May indicate a high concentration of inorganic materials, which could be desirable or undesirable depending on the application. For example, in food products, high ash content might suggest a high mineral content, which could be beneficial or detrimental depending on the specific minerals present.
- Low Ash Content: Suggests a low concentration of inorganic materials, which might be preferred in certain applications where purity is critical.
In summary, ash content is a measure of the inorganic, noncombustible material in a sample. It is determined by burning the sample and measuring the residue left behind, which consists primarily of inorganic compounds. Understanding ash content is crucial in various fields for quality control, nutritional analysis, and environmental assessment.
Summary Table:
| Aspect | Details |
|---|---|
| Definition | Inorganic residue left after burning a sample. |
| Organic Components | Combustible carbon-based compounds oxidized into gases. |
| Inorganic Components | Non-combustible materials like metal oxides and salts. |
| Measurement Process | Combustion → Residue Collection → Analysis. |
| Applications | Quality control, nutritional analysis, environmental science. |
| Factors Affecting Ash | Sample composition, combustion conditions. |
| Implications | High ash: high inorganic content; Low ash: low inorganic content. |
Need help analyzing ash content in your samples? Contact our experts today!
Visual Guide
Related Products
- 1700℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
People Also Ask
- How accurate is the muffle furnace? Achieve ±1°C Control and ±2°C Uniformity
- What are the different types of laboratory furnaces? Find the Perfect Fit for Your Application
- What is the difference between a box furnace and a muffle furnace? Choose the Right Lab Furnace for Your Application
- What is done by ashing in muffle furnace? A Guide to Precise Inorganic Content Analysis
- What temperature should a muffle furnace be for ash content? Achieve Accurate Results with the Right Heat



















