In essence, sintering is the critical manufacturing step that transforms a fragile, pressed powder compact into a strong, coherent, and dense solid part. By applying heat below the material's melting point, sintering initiates atomic diffusion between particles, creating powerful metallurgical bonds, reducing internal voids, and fundamentally changing the material's mechanical and physical properties.
Sintering should not be viewed as simple heating. It is a controlled process of atomic-level fusion that fundamentally converts a weakly-held collection of particles into a solid, engineered material with significantly enhanced density and strength.

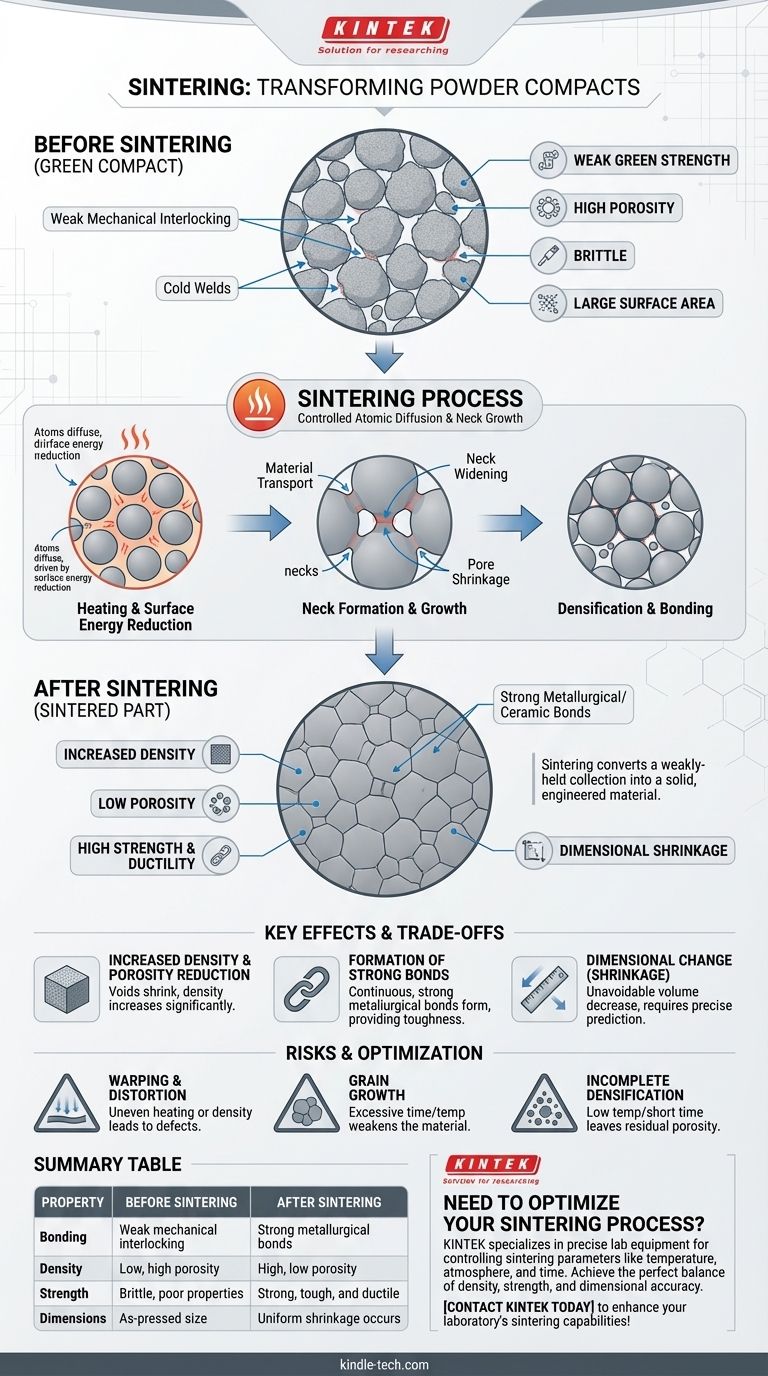
From Fragile Compact to Solid Part: The Core Transformation
To understand the effects of sintering, we must first appreciate the state of the material before the process begins. The goal is to move from a weak preliminary form to a robust final product.
The "Green" Compact
After pressing, the powder forms a shape known as a "green" compact. The particles are held together only by mechanical interlocking and weak "cold welds" formed at their contact points under pressure.
This green compact has enough structural integrity, or "green strength," to be handled, but it is brittle and has poor mechanical properties.
The Driving Force: Reducing Surface Energy
The fundamental driver for sintering is the reduction of surface energy. A fine powder has an enormous amount of surface area, which is an energetically unfavorable state.
Heating provides the thermal energy necessary for atoms to move. The system naturally seeks a lower energy state by reducing this surface area, much like how small soap bubbles merge to form larger ones.
The Mechanism: Atomic Diffusion and Neck Growth
At sintering temperature, atoms begin to diffuse across the boundaries of adjacent particles. This material transport causes "necks" to form and grow at the contact points.
These necks are the initial metallurgical bonds. As the process continues, these necks widen, pulling the particle centers closer together and gradually eliminating the pores between them.
The Primary Physical and Mechanical Changes
The atomic-level changes initiated by sintering result in several critical macroscopic effects on the powder compact.
Increased Density and Porosity Reduction
As material flows from the particles to form and grow the necks, the empty space, or voids, between the particles begins to shrink and close up.
This process directly leads to a significant increase in the overall density of the part. A well-sintered component will have much lower porosity than its green compact precursor.
Formation of Strong Metallurgical Bonds
The cold welds of the green compact are replaced by continuous, strong metallurgical or ceramic bonds across particle boundaries.
This transformation is the primary source of the part's final strength, toughness, and ductility. The individual particles effectively become a single, solid mass.
Dimensional Change (Shrinkage)
A direct and crucial consequence of increased density is a decrease in the overall volume of the part. This phenomenon is known as shrinkage.
This change in dimensions is unavoidable and must be carefully predicted and controlled to ensure the final part meets its required geometric tolerances.
Understanding the Inevitable Trade-offs and Risks
While sintering is essential for creating strong parts, the process is not without challenges. Achieving the desired properties requires balancing competing factors and mitigating potential defects.
The Challenge of Shrinkage
While necessary for densification, shrinkage must be uniform and predictable. Uneven heating or inconsistencies in the green compact's density can lead to warping or distortion.
The Risk of Coarse Grains
Holding a material at a high temperature for too long can lead to excessive grain growth. Large grains can often weaken the material, reducing its strength and making it more brittle.
Optimizing sintering involves achieving full density while minimizing this unwanted grain growth, a balance often controlled by time and temperature.
Potential for Incomplete Densification
If the temperature is too low or the time is too short, the pores between particles may not fully close. This residual porosity can act as a stress concentration point, significantly weakening the final mechanical properties of the part.
Controlling Sintering for Your Desired Outcome
The specific parameters of the sintering cycle—temperature, time, and atmosphere—are chosen to achieve a specific set of material properties.
- If your primary focus is maximum strength and density: Aim for higher temperatures and sufficient time to allow for the near-complete elimination of pores, but monitor closely to prevent excessive grain growth.
- If your primary focus is precise dimensional control: You must meticulously characterize your powder and pressing process to accurately predict and compensate for shrinkage.
- If your primary focus is creating a porous material (e.g., for filters): Use lower temperatures or shorter times to encourage neck formation for strength without fully closing the pore network.
Ultimately, mastering the sintering process is about precisely controlling this atomic-level transformation to engineer the final properties of your material.
Summary Table:
| Property | Before Sintering (Green Compact) | After Sintering |
|---|---|---|
| Bonding | Weak mechanical interlocking | Strong metallurgical bonds |
| Density | Low, high porosity | High, low porosity |
| Strength | Brittle, poor mechanical properties | Strong, tough, and ductile |
| Dimensions | As-pressed size | Uniform shrinkage occurs |
Need to optimize your sintering process for stronger, more reliable parts? KINTEK specializes in the precise lab equipment and consumables necessary to control sintering parameters like temperature, atmosphere, and time. Our solutions help you achieve the perfect balance of density, strength, and dimensional accuracy for your materials. Contact our experts today to discuss how we can enhance your laboratory's sintering capabilities!
Visual Guide
Related Products
- Vacuum Hot Press Furnace Machine Heated Vacuum Press
- Vacuum Hot Press Furnace Heated Vacuum Press Machine Tube Furnace
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Heated Hydraulic Press Machine with Heated Plates for Vacuum Box Laboratory Hot Press
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
People Also Ask
- What type of furnace is used for calcination? Choose the Right Heating Method for Your Process
- What is the primary function of a high-temperature furnace in thermal stability testing? Ensure Inhibitor Performance
- How does a vacuum unit system contribute to the stainless steel nitriding process? Mastering Plasma Environment Control
- Why is temperature control critical during the condensation and crystallization phase of magnesium vapor? Ensure Safety
- What is the temperature and holding time for sintering? Master the Variables for Optimal Results
- Why is a high-vacuum high-temperature furnace required for the annealing treatment of Ni-SiOC nanocomposites?
- What is the difference between liquid and gas carburizing? Precision, Safety & Environmental Impact
- What is the difference between a batch furnace and a continuous casting furnace? Choose the Right Furnace for Your Production Line



















