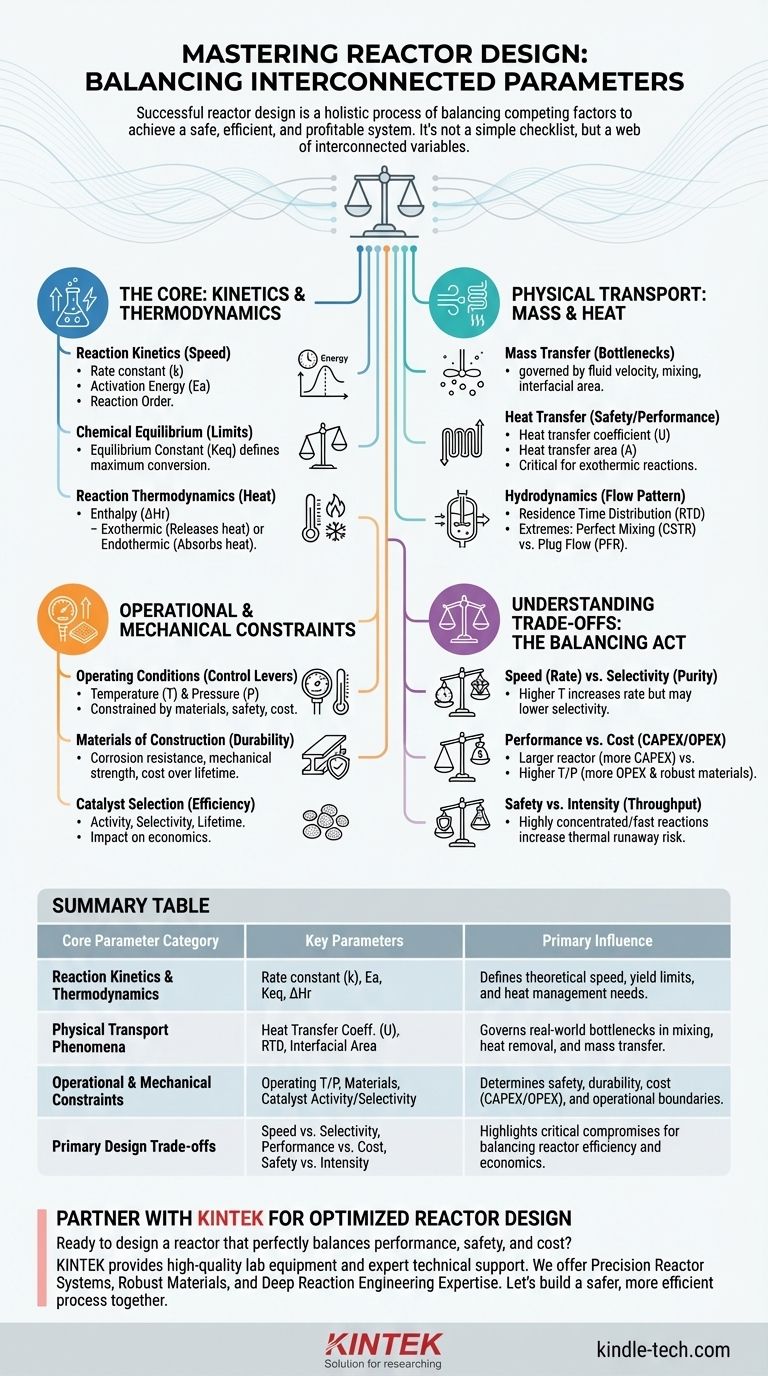
In reactor design, the key parameters are not a simple checklist but a web of interconnected variables that define the system's performance, safety, and cost. At its core, design involves defining the reaction kinetics, managing heat and mass transfer, controlling the fluid dynamics, and selecting appropriate operating conditions and materials. Each choice directly influences the others, requiring a holistic approach to achieve the desired outcome.
Successful reactor design is less about optimizing individual parameters and more about finding the optimal balance between competing factors. The goal is to create a system where kinetics, transport phenomena, and operational constraints work in concert to achieve a specific processing objective safely and economically.

The Core Chemical Reaction: Kinetics and Thermodynamics
The reaction itself is the heart of the process. Understanding its fundamental characteristics is the non-negotiable first step, as it dictates the theoretical limits of your system.
Reaction Kinetics
This describes how fast a reaction proceeds. The rate equation is the primary parameter, which includes the rate constant (k), reaction order, and activation energy (Ea). These collectively determine how reaction speed changes with concentration and temperature.
Chemical Equilibrium
For reversible reactions, the equilibrium constant (Keq) defines the maximum possible conversion you can achieve under given conditions. This parameter tells you the theoretical ceiling for your process yield, which no amount of reactor engineering can overcome.
Reaction Thermodynamics
This concerns the heat effects of the reaction. The enthalpy of reaction (ΔHr) is the critical parameter, indicating whether a reaction is exothermic (releases heat) or endothermic (absorbs heat). This dictates the entire heat management strategy for the reactor.
Physical Transport Phenomena: Moving Mass and Heat
A reaction can only proceed as fast as reactants can be brought together and products can be removed. These physical transport processes often become the real-world bottleneck.
Mass Transfer Limitations
Reactants must move from the bulk fluid to the reaction site (e.g., a catalyst surface). This rate is governed by factors like fluid velocity, mixing intensity, and interfacial area. If mass transfer is slow, your reactor will underperform even with fast intrinsic kinetics.
Heat Transfer Requirements
Managing temperature is arguably the most critical aspect of reactor safety and performance. The key parameters are the overall heat transfer coefficient (U) and the heat transfer area (A). For exothermic reactions, you must be able to remove heat faster than it is generated to prevent a dangerous "runaway" condition.
Hydrodynamics and Mixing
The flow pattern within the reactor determines how long different fluid elements spend inside it. This is characterized by the residence time distribution (RTD). The two ideal extremes are perfect mixing (modeled by a Continuous Stirred-Tank Reactor, or CSTR) and plug flow with no axial mixing (modeled by a Plug Flow Reactor, or PFR).
Operational and Mechanical Constraints
These parameters bridge the gap between chemical engineering theory and real-world implementation. They define the physical and operational boundaries of the reactor.
Operating Temperature and Pressure
These are the primary control levers for influencing reaction rate and equilibrium. However, they are constrained by material limits, safety considerations, and economic trade-offs (e.g., high pressure is expensive to contain).
Materials of Construction
The chosen material must withstand the process's temperature, pressure, and corrosivity for the lifetime of the plant. Parameters here include corrosion resistance, mechanical strength at operating temperature, and cost.
Catalyst Selection and Deactivation
For catalyzed reactions, the catalyst is a central design parameter. Its activity (speed), selectivity (directing to desired products), and lifetime (resistance to deactivation) have a profound impact on process economics.
Understanding the Trade-offs: A Balancing Act
Every design choice involves a compromise. Recognizing these trade-offs is the mark of an experienced technical professional.
Speed vs. Selectivity
Increasing temperature often boosts the reaction rate, but it can also accelerate undesirable side reactions, lowering selectivity and yield. This creates a trade-off between throughput and product purity.
Performance vs. Cost
A larger reactor provides a longer residence time, potentially increasing conversion. However, this increases capital expenditure (CAPEX). Similarly, operating at higher pressures or temperatures may improve performance but requires more robust, expensive materials and higher operating expenditure (OPEX).
Safety vs. Intensity
Process intensification—getting more product from a smaller volume—is a major economic driver. However, running a highly concentrated, fast, exothermic reaction increases the risk and severity of a potential thermal runaway. A robust safety design is paramount.
Making the Right Choice for Your Goal
Your reactor design must be driven by your primary objective. There is no single "best" reactor, only the best reactor for a specific task.
- If your primary focus is maximizing conversion for a slow reaction: A reactor providing long residence time, like a large Batch reactor or a long Plug Flow Reactor (PFR), is the logical choice.
- If your primary focus is precise temperature control for a highly exothermic process: A reactor with a high surface-area-to-volume ratio is essential. This could be a CSTR with an extensive cooling jacket or a PFR made of small-diameter tubes.
- If your primary focus is high-volume, continuous production: A continuous system like a CSTR or PFR is almost always preferred over a batch process for its efficiency and consistency at scale.
- If your primary focus is versatility for multiple products or low initial cost: A simple jacketed Batch reactor often provides the most flexibility with the lowest initial capital investment.
Ultimately, successful reactor design is a holistic process where each parameter is evaluated in relation to the others to create a safe, efficient, and profitable system.
Summary Table:
| Core Parameter Category | Key Parameters | Primary Influence |
|---|---|---|
| Reaction Kinetics & Thermodynamics | Rate constant (k), Activation Energy (Ea), Equilibrium Constant (Keq), Enthalpy (ΔHr) | Defines theoretical speed, yield limits, and heat management needs. |
| Physical Transport Phenomena | Heat Transfer Coefficient (U), Residence Time Distribution (RTD), Interfacial Area | Governs real-world bottlenecks in mixing, heat removal, and mass transfer. |
| Operational & Mechanical Constraints | Operating Temperature/Pressure, Materials of Construction, Catalyst Activity/Selectivity | Determines safety, durability, cost (CAPEX/OPEX), and operational boundaries. |
| Primary Design Trade-offs | Speed vs. Selectivity, Performance vs. Cost, Safety vs. Intensity | Highlights critical compromises for balancing reactor efficiency and economics. |
Ready to design a reactor that perfectly balances performance, safety, and cost for your specific process?
At KINTEK, we specialize in providing the high-quality lab equipment and expert support needed to optimize your reactor design. Whether you're scaling up a reaction, managing exothermic processes, or selecting the right materials, our team can help you navigate the complex trade-offs to achieve your goals.
We provide:
- Precision Reactor Systems: From bench-top to pilot-scale, designed for excellent heat and mass transfer.
- Robust Materials & Components: Ensuring safety and longevity under demanding conditions.
- Expert Technical Support: Leverage our deep understanding of reaction engineering to make informed decisions.
Let's build a safer, more efficient process together. Contact our experts today to discuss your reactor design challenges!
Visual Guide
Related Products
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- Electric Split Lab Cold Isostatic Press CIP Machine for Cold Isostatic Pressing
People Also Ask
- How does a high-pressure hydrothermal reactor facilitate waste mushroom substrate utilization? Boost Hydrochar Yields
- What function does a high-pressure reactor serve in hydrothermal synthesis? Mastering Mesoporous Material Control
- What is a high pressure reactor? Unlock Chemical Reactions with Precision Control
- What are the considerations for bioreactor design? Optimize Your Bioprocess for Maximum Yield
- How do precision reaction vessels and heaters ensure product quality for High-Entropy Alloy nanoparticles?
- Why must HTL reactors have high corrosion resistance? Ensure Safety in Hydrothermal Liquefaction
- What are the material requirements for reaction vessels using sodium hydroxide? Simplified Lab Equipment Solutions
- What specific reaction environment does a high-pressure reactor provide for HDS? Optimize Your Fuel Purification



















