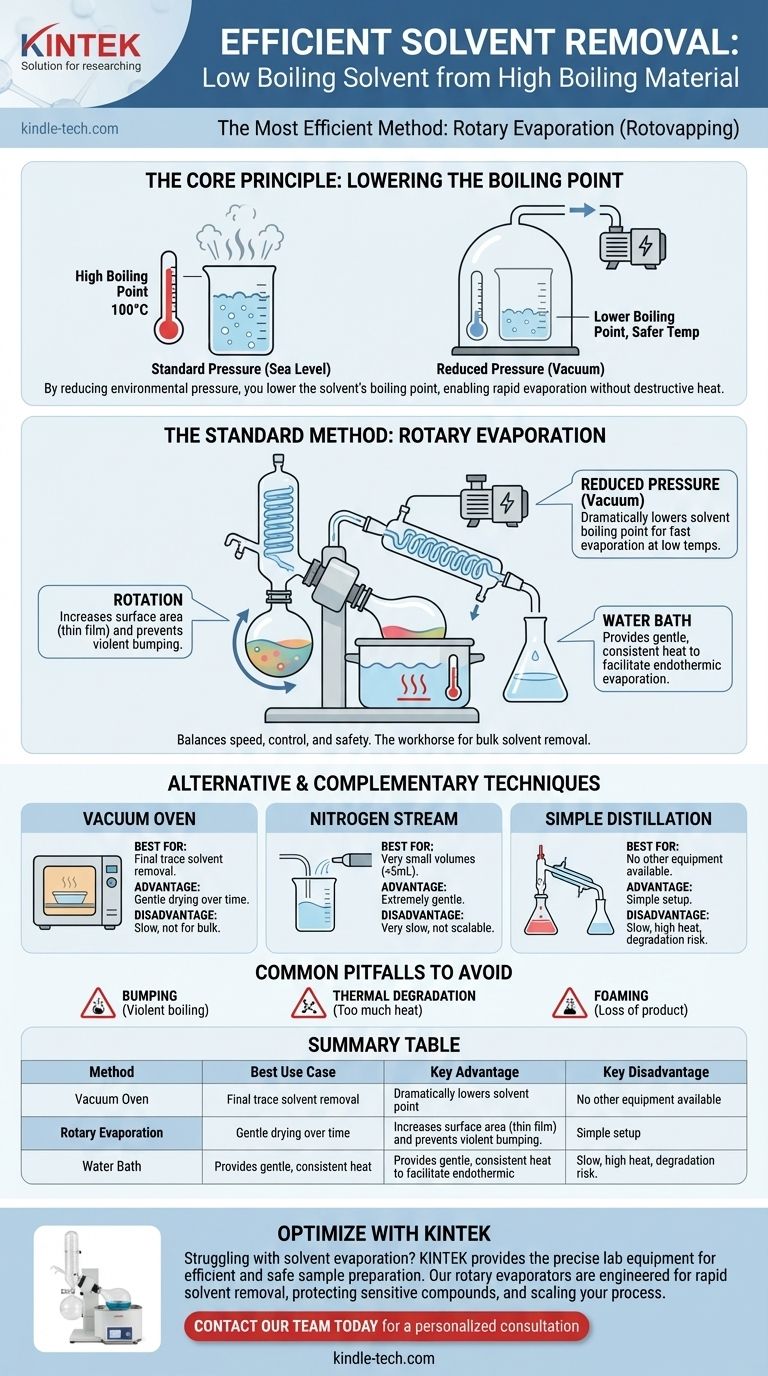
In most laboratory and production settings, the most efficient method for removing a low boiling point solvent from a high boiling point material is rotary evaporation. This technique, often called "rotovapping," combines reduced pressure, gentle heating, and flask rotation to rapidly and safely remove volatile solvents without damaging the desired, less volatile compound. It is the standard for its balance of speed, control, and effectiveness.
The core challenge is not simply to heat the mixture, but to remove the solvent quickly without causing thermal degradation to your high-boiling point product. The most efficient solutions work by lowering the solvent's boiling point via reduced pressure, enabling rapid evaporation at a much lower, safer temperature.

The Core Principle: Lowering the Boiling Point
To understand why certain methods are superior, you must first grasp the physics of evaporation. Efficiency comes from manipulating the environment, not just applying brute-force heat.
How Pressure Affects Boiling
A liquid boils when its vapor pressure equals the pressure of the environment above it. At sea level, water boils at 100°C because that is the temperature at which its vapor pressure matches standard atmospheric pressure.
If you reduce the environmental pressure—for example, by using a vacuum pump—you lower the threshold the vapor pressure needs to reach. This means the liquid will boil at a significantly lower temperature.
The Goal: Gentle Yet Rapid Evaporation
The objective is to make the solvent boil away while your high-boiling material remains a liquid or solid. By pulling a vacuum, you can often make a solvent like acetone (normal boiling point: 56°C) boil at or below room temperature. This minimizes the risk of thermal degradation to your target compound.
The Standard Method: Rotary Evaporation
A rotary evaporator (rotovap) is specifically designed to exploit the principle of reduced-pressure evaporation. It is the workhorse of synthetic chemistry labs for this exact task.
The Role of Reduced Pressure
The system is sealed and connected to a vacuum source. This dramatically lowers the solvent's boiling point, allowing for very fast evaporation without excessive heat.
The Advantage of Rotation
The flask containing the mixture is continuously rotated. This has two critical benefits:
- Increased Surface Area: Rotation constantly spreads the mixture as a thin film on the inner wall of the flask, maximizing the surface area available for evaporation. This is far more efficient than boiling from a static pool of liquid.
- Prevents Bumping: Boiling under vacuum can be violent, causing the solution to "bump" or splash uncontrollably. The smooth, constant agitation from rotation largely prevents this, ensuring your product isn't lost.
The Importance of the Water Bath
The rotovap flask is partially submerged in a heated water or oil bath. This provides a gentle and consistent energy source to facilitate the evaporation, which is an endothermic (energy-consuming) process. The key is that the bath temperature can be kept low—often just 30-40°C—protecting your product.
Alternative and Complementary Techniques
While rotary evaporation is the most common and efficient tool for bulk solvent removal, other methods are used in specific situations.
Simple or Fractional Distillation
This is the classic method taught in introductory chemistry. The mixture is heated until the low-boiling solvent vaporizes, travels through a condenser, and is collected in a separate flask.
This method is far less efficient for this purpose because it relies solely on high temperatures, is much slower, and carries a greater risk of degrading heat-sensitive compounds.
Vacuum Oven
A vacuum oven is excellent for removing the final, trace amounts of solvent after the bulk has been removed by another method (like a rotovap). By placing your material on a dish inside a heated, evacuated chamber, you can gently draw out stubborn, residual solvent over several hours or days.
Nitrogen/Inert Gas Stream
For very small volumes or extremely sensitive materials, you can gently blow a stream of dry nitrogen or argon over the surface of the liquid. This works by constantly displacing the solvent-saturated air just above the liquid's surface, disrupting the equilibrium and encouraging further evaporation. It is gentle but slow and only suitable for small scales.
Understanding the Pitfalls and Trade-offs
Efficiency is not just about speed; it's about preserving your final product. Be aware of these common issues.
The Risk of "Bumping"
As mentioned, sudden, violent boiling under vacuum is a real risk, especially without rotation. This can cause you to lose a significant portion of your product into the vacuum system. Always apply vacuum gradually and ensure smooth agitation.
Product Thermal Stability
Just because your material has a high boiling point does not mean it is stable at high temperatures. Many complex organic molecules can begin to decompose at temperatures far below their boiling point. The goal is always to use the lowest possible temperature that allows for efficient evaporation.
Dealing with Foaming
Some mixtures, particularly oils or solutions containing surfactants, are prone to foaming under vacuum. This can carry your product out of the flask. Using a larger flask, applying vacuum very slowly, or using an anti-foaming agent can mitigate this. A "bump trap" between your flask and the rotovap is essential protection.
Making the Right Choice for Your Goal
Select your method based on the scale of your work and the stage of your purification process.
- If your primary focus is bulk solvent removal (10 mL to several liters): Use a rotary evaporator. It provides the best combination of speed, control, and safety for your product.
- If your primary focus is removing the final trace amounts of solvent: Use a vacuum oven after the bulk of the solvent is already gone.
- If your primary focus is removing solvent from a very small sample (<5 mL): A gentle stream of inert gas or a small-scale vacuum setup (like a "high vac" line) is often sufficient and practical.
- If you lack specialized equipment: Simple distillation can work, but you must monitor the temperature carefully and accept that it will be a slow process with a higher risk to your product.
Ultimately, mastering solvent removal is about using pressure to your advantage, allowing you to achieve rapid evaporation without resorting to destructive heat.
Summary Table:
| Method | Best Use Case | Key Advantage | Key Disadvantage |
|---|---|---|---|
| Rotary Evaporation | Bulk removal (10mL to liters) | Fast, gentle, prevents bumping | Requires specialized equipment |
| Vacuum Oven | Final trace solvent removal | Gentle drying over time | Slow, not for bulk removal |
| Nitrogen Stream | Very small volumes (<5mL) | Extremely gentle | Very slow, not scalable |
| Simple Distillation | When no other equipment is available | Simple setup | Slow, high thermal degradation risk |
Optimize Your Solvent Removal Process with KINTEK
Struggling with solvent evaporation that risks degrading your valuable high-boiling point materials? KINTEK specializes in the precise lab equipment you need for efficient and safe sample preparation.
Our range of rotary evaporators (rotovaps) are engineered to provide the perfect balance of reduced pressure, gentle heating, and continuous rotation, enabling you to:
- Remove solvents rapidly without applying destructive heat.
- Protect sensitive compounds from thermal degradation.
- Scale your processes from small-scale R&D to larger production volumes.
Whether you need a standard rotovap for your chemistry lab or a specialized system for challenging applications, KINTEK has the solution. Our experts are ready to help you select the ideal equipment for your specific solvents and materials.
Enhance your lab's efficiency and protect your products—contact our team today for a personalized consultation!
Visual Guide
Related Products
- Circulating Water Vacuum Pump for Laboratory and Industrial Use
- Molybdenum Tungsten Tantalum Evaporation Boat for High Temperature Applications
- Ceramic Evaporation Boat Set Alumina Crucible for Laboratory Use
- Laboratory Disc Rotary Mixer for Efficient Sample Mixing and Homogenization
- Hemispherical Bottom Tungsten Molybdenum Evaporation Boat
People Also Ask
- How is a circulating water vacuum pump utilized for hydrogen production residues? Optimize Your Solid-Liquid Separation
- What is the importance of a vacuum pump for Schottky hybrid interfaces? Achieve Atomic-Level Purity and Bonding
- What is the purpose of the compression chamber in a vacuum pump? The Heart of Vacuum Generation
- How does the impeller rotation affect the gas flow in a water circulating vacuum pump? A Guide to the Liquid Ring Principle
- What are the advantages of a water circulating vacuum pump? Superior Durability for Demanding Lab Environments



















