Critically, there is no single operating pressure for a reactor. This value is not a universal constant but a fundamental design parameter that is entirely dictated by the specific chemical process it is built to contain. Reactor pressures can range from a high vacuum to thousands of atmospheres, depending on the requirements of the reaction kinetics, thermodynamics, and desired product state.
A reactor's operating pressure is a deliberate engineering choice, not an inherent property. It is determined by the chemistry of the reaction and fundamentally influences the vessel's design, materials, cost, and safety requirements.
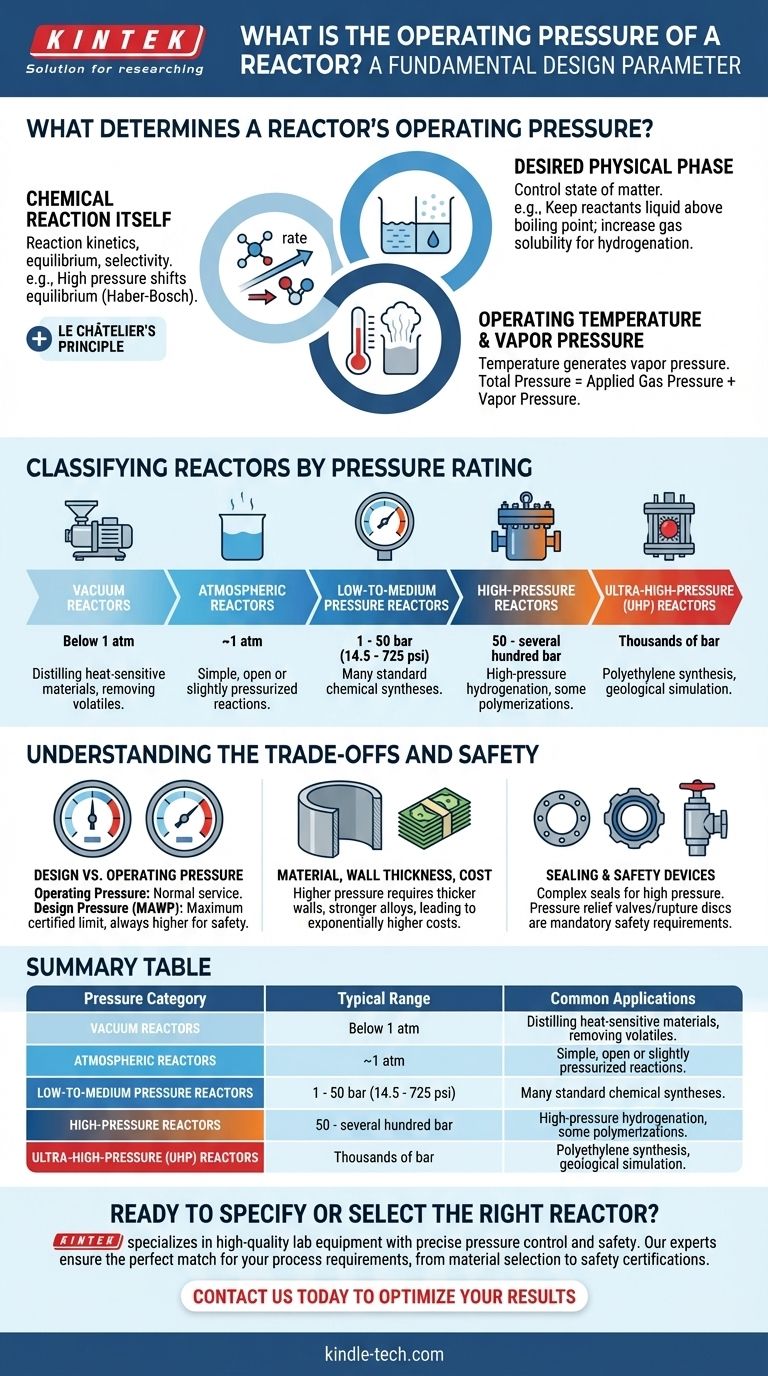
What Determines a Reactor's Operating Pressure?
The required pressure for a chemical process is a result of several interconnected physical and chemical factors. Engineers carefully calculate these needs before a reactor is ever built.
The Chemical Reaction Itself
The primary driver is the nature of the reaction. Many chemical reactions are sensitive to pressure, which can influence reaction rate, equilibrium, and selectivity.
For example, in ammonia synthesis (Haber-Bosch process), high pressure is used to shift the chemical equilibrium toward the product side, dramatically increasing the yield. This is an application of Le Châtelier's principle.
Desired Physical Phase
Pressure is a powerful tool for controlling the state of matter. A key goal is often to keep reactants in a liquid phase above their normal boiling points or to increase the solubility of a gas in a liquid.
In hydrogenation reactions, high hydrogen pressure is required to dissolve enough gas into the liquid solvent to allow the reaction to proceed efficiently at the catalyst's surface.
Operating Temperature and Vapor Pressure
For any sealed reactor containing a liquid, the operating temperature will generate a corresponding vapor pressure. As the temperature increases, the pressure inside the sealed vessel increases naturally.
This must be accounted for in the reactor's design. The total operating pressure will be the sum of any applied gas pressure plus the vapor pressure of the liquids and reactants at the operating temperature.
Classifying Reactors by Pressure Rating
While every reactor is designed for a specific pressure, they can be grouped into general categories.
Vacuum Reactors
These reactors operate below atmospheric pressure. A vacuum is used to lower the boiling point of liquids, which is useful for distilling heat-sensitive materials or removing volatile byproducts.
Atmospheric Reactors
The simplest category, these vessels are not designed to handle significant pressure or vacuum. They are often open to the atmosphere or operate at a very slight positive pressure to prevent air from entering.
Low-to-Medium Pressure Reactors
This is a broad and common category in industry, often ranging from just above atmospheric pressure up to around 50 bar (725 psi). Many standard chemical syntheses fall within this range.
High-Pressure Reactors
These are highly specialized vessels designed for pressures from 50 bar up to several hundred bar. They require thick walls, specialized sealing mechanisms, and robust safety systems. Applications include high-pressure hydrogenation and some polymerization processes.
Ultra-High-Pressure (UHP) Reactors
Operating at thousands of bar, these are at the extreme end of engineering. They are used for niche applications like synthesizing polyethylene or in research for simulating geological conditions.
Understanding the Trade-offs and Safety
Choosing or designing for a specific pressure involves critical engineering trade-offs and safety considerations.
Design Pressure vs. Operating Pressure
These two terms are not interchangeable. Operating pressure is the pressure during normal service. Design pressure (or MAWP - Maximum Allowable Working Pressure) is the maximum pressure the vessel is certified to handle safely. The design pressure is always set higher than the operating pressure to provide a crucial safety margin.
Material, Wall Thickness, and Cost
As design pressure increases, the required wall thickness of the reactor increases dramatically. This necessitates using stronger, often more exotic and expensive, alloys. The cost of a reactor increases exponentially with its pressure rating.
Sealing and Safety Devices
Low-pressure reactors can use simple gaskets. High-pressure systems require complex, precision-engineered seals. Furthermore, all pressurized reactors are legally required to have safety devices like pressure relief valves or rupture discs that prevent catastrophic failure in an overpressure event.
Making the Right Choice for Your Goal
Selecting or specifying a reactor's pressure is about matching the equipment to the process requirements.
- If your primary focus is designing a new chemical process: Your decision must be driven by the reaction kinetics, thermodynamics, and phase requirements needed to maximize yield and safety.
- If your primary focus is selecting an existing reactor for a task: You must ensure the reactor's Design Pressure (MAWP) is safely above your required Operating Pressure, accounting for all potential temperature and reaction excursions.
- If your primary focus is operational safety: You must know the reactor's design pressure and ensure protective devices are correctly set, certified, and maintained to prevent exceeding it under any circumstances.
Ultimately, a reactor's pressure is the single most important parameter defining its construction and safe operational limits.
Summary Table:
| Pressure Category | Typical Range | Common Applications |
|---|---|---|
| Vacuum Reactors | Below 1 atm | Distilling heat-sensitive materials |
| Atmospheric Reactors | ~1 atm | Simple, open or slightly pressurized reactions |
| Low-to-Medium Pressure | 1 - 50 bar | Many standard chemical syntheses |
| High-Pressure Reactors | 50 - several hundred bar | High-pressure hydrogenation, polymerization |
| Ultra-High-Pressure (UHP) | Thousands of bar | Polyethylene synthesis, geological simulation |
Ready to specify or select the right reactor for your chemical process?
At KINTEK, we specialize in high-quality lab equipment, including reactors designed for precise pressure control and safety. Our expertise ensures you get a vessel that perfectly matches your process requirements—from material selection to safety certifications.
Contact us today to discuss your application and let our experts help you achieve optimal results with the right equipment. Get in touch now!
Visual Guide
Related Products
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Manual High Temperature Heated Hydraulic Press Machine with Heated Plates for Lab
People Also Ask
- How does a hydrothermal reactor prepare mesoporous hydroxyapatite? Unlock Superior Catalyst Synthesis
- What controls the rate of reactions? Master the 5 Key Factors for Optimal Chemical Processes
- Why is high corrosion resistance a critical requirement for reaction equipment? Ensure Purity in Acid Hydrolysis
- Why are industrial-grade high-pressure reactors necessary for the AFEX process? Unlock Biomass Conversion Potential
- What are the advantages of using water and organic solvent biphasic system reactors for furfural research? Maximize Yield
- What is a high pressure reactor? Unlock Chemical Reactions with Precision Control
- What role does a hydrothermal reactor play in porous hydroxyapatite synthesis? Optimize HA Catalysts with Precision
- Why are PTFE-lined reactors critical for MOF synthesis? Ensure High Purity and Corrosion Resistance in Your Lab



















