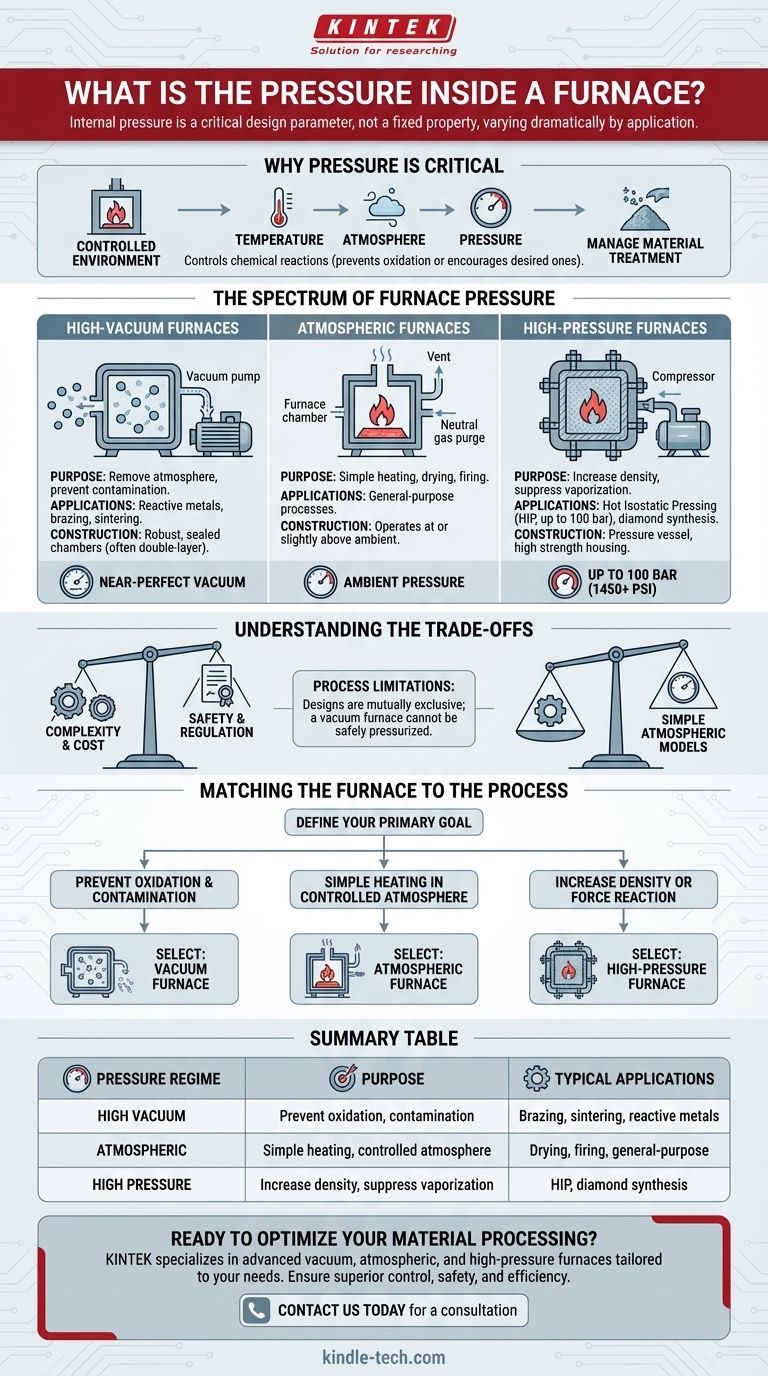
There is no single pressure inside a furnace. Instead, the internal pressure is a critical design parameter that varies dramatically depending on the furnace's specific application. It can range from a near-perfect vacuum to pressures exceeding 100 times that of the normal atmosphere.
The internal pressure of a furnace is not a fixed property but a deliberately engineered environment. It is controlled to achieve specific material outcomes, from preventing oxidation in a vacuum to increasing density under high pressure.
Why Pressure is a Critical Variable
A furnace is more than just a hot box; it is a controlled environment. Pressure, along with temperature and atmospheric composition, is one of the fundamental variables used to precisely manage a material treatment process.
Controlling the pressure allows operators to prevent unwanted chemical reactions, such as oxidation from atmospheric oxygen, or to encourage desired ones that only occur under specific pressure conditions.
The Spectrum of Furnace Pressure
Furnaces are generally designed to operate within one of three pressure regimes: vacuum, atmospheric, or high pressure. Each serves a distinct purpose and requires vastly different construction.
High-Vacuum Furnaces
The purpose of a vacuum furnace is to remove the atmosphere from the processing chamber. This is critical for preventing contamination and unwanted reactions when working with reactive metals or for processes like brazing and sintering.
These systems use vacuum pumps to lower the internal pressure far below that of the outside atmosphere. Their construction requires robust, perfectly sealed chambers, often using a double-layer shell structure to maintain the vacuum integrity.
High-Pressure Furnaces
At the other extreme, high-pressure furnaces are used to create conditions far above normal atmospheric pressure. This can be used to increase material density, suppress the vaporization of volatile elements, or synthesize materials like artificial diamonds.
For example, a graphite furnace used for processes like Hot Isostatic Pressing (HIP) can achieve an internal pressure of up to 100 bar (over 1,450 PSI). The ability to contain such pressure is determined entirely by the strength of the furnace housing.
Atmospheric Furnaces
The most common and simplest furnaces operate at or slightly above ambient atmospheric pressure.
These are used for general-purpose heating, drying, or firing processes where interaction with the air is either acceptable or managed by purging the chamber with a neutral gas like argon or nitrogen.
Understanding the Trade-offs
Choosing a furnace with specific pressure capabilities involves significant trade-offs in cost, complexity, and safety.
Complexity and Cost
Achieving either a deep vacuum or a high positive pressure requires sophisticated engineering. The need for vacuum pumps, heavy-duty seals, and reinforced chambers makes these furnaces significantly more expensive to build and operate than simple atmospheric models.
Safety and Regulation
High-pressure furnaces are classified as pressure vessels. They are subject to stringent safety regulations and certification requirements to prevent catastrophic failure, which adds a significant layer of operational and maintenance overhead.
Process Limitations
A furnace is purpose-built for its pressure regime. A furnace designed to hold a vacuum cannot be safely pressurized, and a high-pressure system is not optimized to create a high-quality vacuum. The design choices are mutually exclusive.
Matching the Furnace to the Process
To select the correct equipment, you must first define your primary processing goal.
- If your primary focus is to prevent oxidation and contamination: You require a vacuum furnace to remove reactive atmospheric gases.
- If your primary focus is to increase material density or force a chemical reaction: You need a high-pressure furnace to create the necessary physical conditions.
- If your primary focus is simple heating in a controlled or ambient atmosphere: A standard atmospheric furnace is the most direct and cost-effective solution.
Understanding the role of pressure transforms the furnace from a simple oven into a precise materials processing tool.
Summary Table:
| Pressure Regime | Purpose | Typical Applications |
|---|---|---|
| High Vacuum | Prevent oxidation and contamination | Brazing, sintering, heat-treating reactive metals |
| Atmospheric | Simple heating with controlled atmosphere | Drying, firing, general-purpose processes |
| High Pressure | Increase density, suppress vaporization | Hot Isostatic Pressing (HIP), diamond synthesis |
Ready to Optimize Your Material Processing?
Choosing the right furnace pressure regime is critical for achieving precise results—whether you need to prevent contamination with a vacuum or enhance density under high pressure. At KINTEK, we specialize in providing advanced lab equipment, including vacuum, atmospheric, and high-pressure furnaces, tailored to your specific research and production needs.
Our experts will help you select the perfect solution to ensure superior process control, safety, and efficiency. Don't leave your outcomes to chance; let KINTEK empower your lab with the right technology.
Contact us today for a personalized consultation!
Visual Guide
Related Products
- Vacuum Hot Press Furnace Heated Vacuum Press Machine Tube Furnace
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- Laboratory High Pressure Vacuum Tube Furnace
People Also Ask
- What are the critical control factors and monitoring limitations in the HPHT process? Master Stability & Efficiency
- What are the advantages of a vacuum hot pressing furnace? Achieve Superior Lithium Niobate Piezoelectric Density
- How does a vacuum hot press furnace address structural defects in as-cast CoCrPtB alloy ingots? Optimize Your Density
- What role does a vacuum hot pressing sintering furnace play in AZ31 densification? Achieve Near-Theoretical Density
- How does a vacuum hot pressing furnace facilitate the preparation of high-density Nb-22.5Cr-5Si alloy bulks? Achieve 99% Density



















