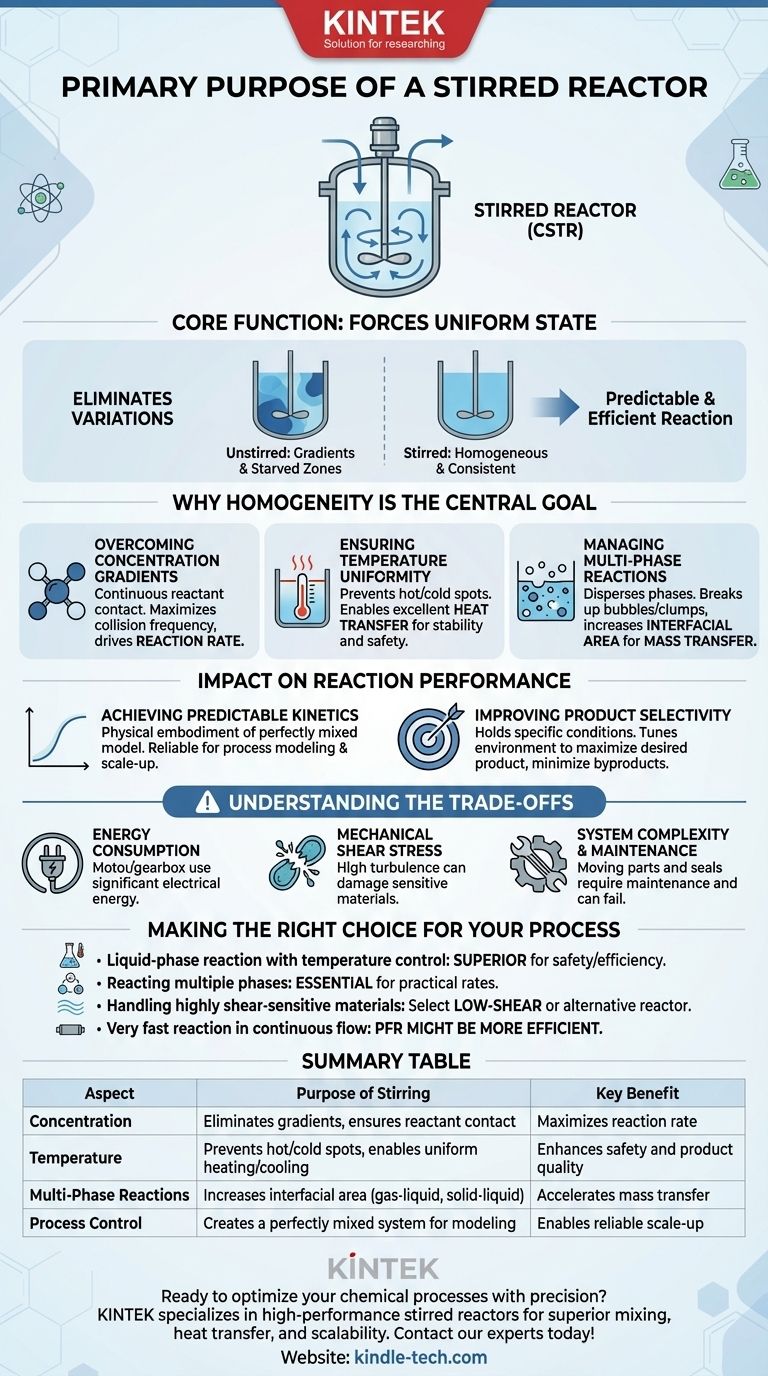
At its core, a stirred reactor's primary purpose is not just to hold chemicals, but to force them into a uniform state. By using a mechanical agitator, it actively eliminates variations in concentration and temperature throughout the reaction volume. This creates a highly controlled and consistent environment, allowing the chemical reaction to proceed as predictably and efficiently as possible.
While any vessel can contain a reaction, a stirred reactor is designed to master it. Its true function is to overcome the physical barriers of heat and mass transfer, ensuring that the chemical kinetics—the speed of the reaction itself—is the only factor limiting the outcome.

Why Homogeneity is the Central Goal
A chemical reaction's success is often dictated by the physical environment in which it occurs. A stirred reactor, often called a Continuous Stirred-Tank Reactor (CSTR) in industrial settings, is engineered to control that environment through mixing.
Overcoming Concentration Gradients
In an unstirred vessel, reactants are consumed locally. This creates zones where the reactant concentration is low, effectively starving the reaction, and other zones where it remains high.
Stirring, or agitation, forcefully moves the bulk fluid. This ensures that fresh reactants are continuously brought into contact, maximizing the collision frequency between molecules and thus driving the reaction rate.
Ensuring Temperature Uniformity
Chemical reactions can be exothermic (release heat) or endothermic (absorb heat). Without mixing, dangerous "hot spots" or inefficient "cold spots" can develop.
Hot spots can degrade your product, cause unwanted side reactions, or even lead to a dangerous thermal runaway. A stirred reactor constantly circulates the fluid past a heating or cooling jacket on the vessel wall, providing excellent heat transfer and maintaining a single, stable temperature.
Managing Multi-Phase Reactions
Many critical reactions involve multiple phases, such as bubbling a gas through a liquid or dissolving a solid catalyst. The reaction can only happen at the interface between these phases.
Stirring is essential for dispersing one phase into another. It breaks up large gas bubbles or solid clumps into fine particles, dramatically increasing the interfacial surface area and accelerating the rate of mass transfer between the phases.
The Impact on Reaction Performance
Controlling the physical environment has a direct and profound impact on the chemical outcome. This is the ultimate reason for using a stirred reactor.
Achieving Predictable Kinetics
Chemical engineers rely on mathematical models to predict and control reaction outcomes. These models almost always assume a perfectly mixed system where properties are uniform.
A well-designed stirred reactor is the physical embodiment of this ideal assumption. This makes process modeling, control, and scale-up from the lab to a production plant far more reliable.
Improving Product Selectivity
For reactions that can produce multiple products, temperature and reactant concentration are often the deciding factors in which product is favored.
By eliminating gradients, a stirred reactor holds the entire system at a specific set of conditions. This allows you to "tune" the environment to maximize the formation of your desired product and minimize the creation of unwanted byproducts, a concept known as improving selectivity.
Understanding the Trade-offs
While powerful, a stirred reactor is not a universal solution. Its design introduces specific challenges that must be managed.
Energy Consumption
The motor and gearbox required to drive the agitator consume significant amounts of electrical energy. This is a primary operational cost, especially for large-scale reactors or when mixing highly viscous fluids.
Mechanical Shear Stress
The impeller's rapid movement creates high fluid velocities and turbulence. While this is good for mixing, the resulting shear forces can damage or destroy sensitive materials. This is a major concern in bioprocessing, where delicate cells or large protein molecules can be easily harmed.
System Complexity and Maintenance
Compared to a simple tank or a pipe reactor (Plug Flow Reactor), a stirred reactor has moving parts: a motor, shaft, bearings, and, most critically, a seal. The seal prevents leakage where the shaft enters the vessel and is a common point of failure and maintenance.
Making the Right Choice for Your Process
The decision to use a stirred reactor depends entirely on the specific demands of your chemical system.
- If your primary focus is a liquid-phase reaction with temperature control: The excellent heat and mass transfer of a stirred reactor makes it the default, superior choice for safety and efficiency.
- If your primary focus is reacting multiple phases (gas-liquid, solid-liquid): Stirring is almost always necessary to create the interfacial area required for the reaction to proceed at a practical rate.
- If your primary focus is handling highly shear-sensitive materials: You must carefully select a low-shear impeller or consider alternative reactor types like a bubble column or airlift reactor.
- If your primary focus is a very fast reaction in a continuous flow: A Plug Flow Reactor (PFR) might be more efficient, as it avoids the back-mixing inherent in a stirred tank.
Ultimately, understanding the role of mixing transforms the reactor from a simple container into a precision instrument for chemical synthesis.
Summary Table:
| Aspect | Purpose of Stirring | Key Benefit |
|---|---|---|
| Concentration | Eliminates gradients, ensures reactant contact | Maximizes reaction rate |
| Temperature | Prevents hot/cold spots, enables uniform heating/cooling | Enhances safety and product quality |
| Multi-Phase Reactions | Increases interfacial area (gas-liquid, solid-liquid) | Accelerates mass transfer |
| Process Control | Creates a perfectly mixed system for modeling | Enables reliable scale-up |
Ready to optimize your chemical processes with precision? KINTEK specializes in high-performance lab equipment, including stirred reactors designed for superior mixing, heat transfer, and scalability. Whether you're developing new reactions or scaling up production, our solutions ensure consistent, reliable results. Contact our experts today to find the perfect reactor for your laboratory needs!
Visual Guide
Related Products
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
People Also Ask
- How does a high-pressure hydrothermal autoclave facilitate the synthesis of BiVO4@PANI nanocomposites? Unlock Precision.
- Why is a High-temperature and High-pressure Autoclave necessary for zirconium alloy testing? Ensure Nuclear Safety.
- What is the function of a high-pressure static autoclave in biomass HTL? Optimize Your Biomass Conversion Research
- What is the significance of the hydrothermal environment in HA preparation? Optimize Mesoporous Structure and Purity
- Why are stainless steel autoclaves key to PCL-TPE preparation? Mastering High-Vacuum Polycondensation



















