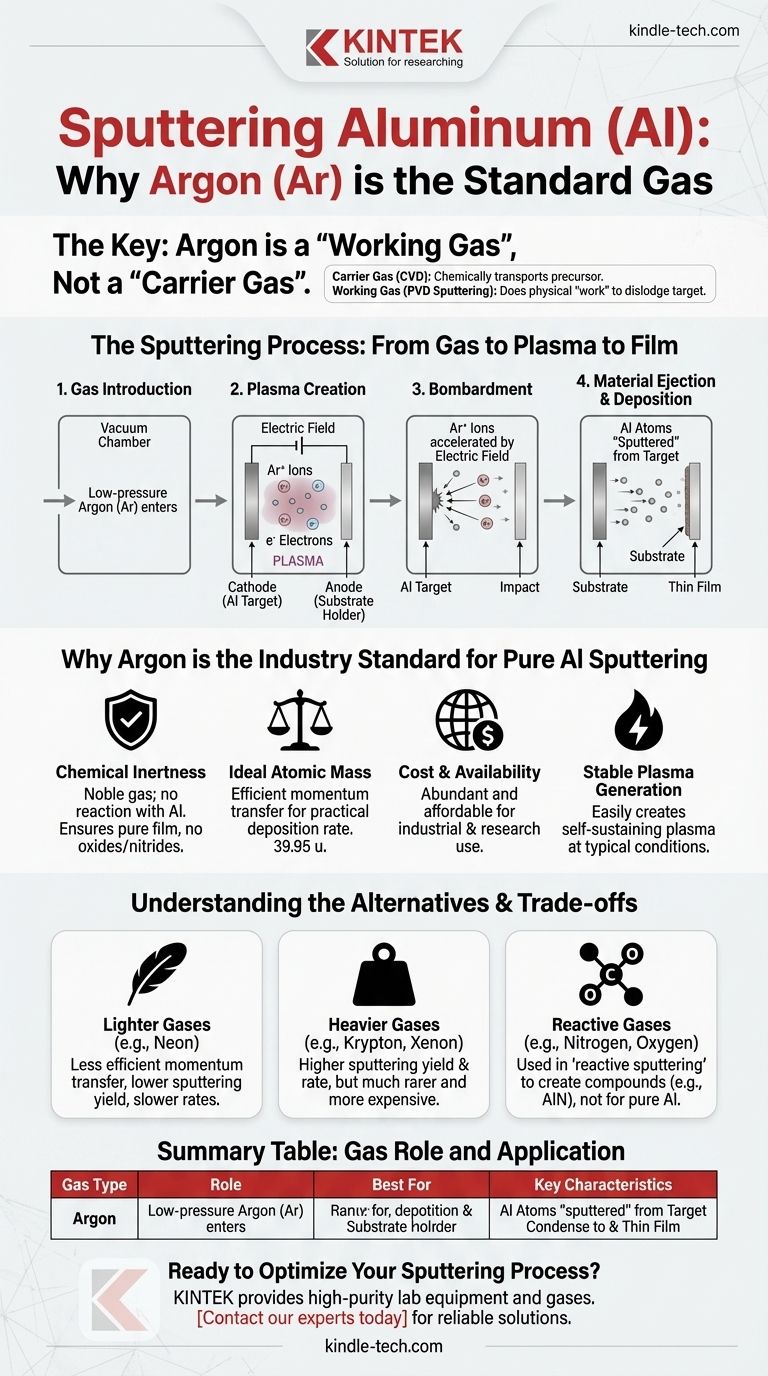
In short, the standard gas used for sputtering aluminum is Argon (Ar). It is not a "carrier gas" in the traditional sense, but rather a "working gas" that is ionized to create a plasma. This plasma is the essential tool that physically removes aluminum atoms from a source target and deposits them onto your substrate.
The term "carrier gas" can be misleading in this context. Argon's role is not to carry aluminum but to act as an energetic projectile. It is ionized into a plasma, and these ions bombard the aluminum target, physically knocking atoms loose for deposition.

The Role of Gas in Sputtering: From Working Gas to Plasma
Understanding the sputtering process begins with understanding why a gas is necessary in the first place. The gas is the medium that enables the entire physical deposition mechanism.
Why "Working Gas" is the Correct Term
A carrier gas, often used in chemical vapor deposition (CVD), chemically transports precursor materials to a surface. In sputtering, a physical vapor deposition (PVD) process, the gas does the physical "work" of dislodging the target material.
Creating the Plasma
The process starts by introducing a low-pressure working gas, like Argon, into a vacuum chamber. A strong electric field is then applied between the aluminum target (cathode) and the substrate holder (anode).
This high voltage energizes the gas, stripping electrons from the Argon atoms and creating a mixture of positive Argon ions (Ar+) and free electrons. This ionized gas is known as a plasma.
The Bombardment Process
The positively charged Argon ions are accelerated by the electric field and slam into the negatively charged aluminum target at high velocity.
Ejecting the Target Material
Each collision transfers kinetic energy from the Argon ion to the aluminum target. If enough energy is transferred, aluminum atoms are physically knocked out, or "sputtered," from the target surface. These ejected aluminum atoms then travel through the vacuum chamber and condense on your substrate, forming a thin film.
Why Argon is the Industry Standard
While other gases can be used, Argon is the overwhelming choice for sputtering pure aluminum for several key reasons. Its properties provide an ideal balance of performance, purity, and cost.
Chemical Inertness
Argon is a noble gas, meaning it is chemically inert. It will not react with the aluminum target or the film being deposited on the substrate. This ensures the final aluminum film is pure and not an unintended oxide or nitride.
Ideal Atomic Mass
Argon's atomic mass (39.95 u) is heavy enough to efficiently transfer momentum and dislodge aluminum atoms (26.98 u) from the target. This results in a practical and controllable deposition rate.
Cost and Availability
Argon is the third-most abundant gas in Earth's atmosphere. Its widespread availability makes it significantly more affordable than other noble gases, which is a critical factor for both industrial production and academic research.
Stable Plasma Generation
Argon has an ionization potential that allows for the creation of a stable, self-sustaining plasma under typical operating pressures and voltages used in sputtering systems.
Understanding the Trade-offs and Alternatives
While Argon is the standard, understanding the alternatives reveals the core principles of the sputtering process. The choice of gas is always a trade-off between deposition rate, cost, and film properties.
Lighter Gases (e.g., Neon)
Lighter noble gases like Neon can be used, but their lower atomic mass results in less efficient momentum transfer. This leads to a significantly lower sputtering yield and slower deposition rates, making them impractical for most applications.
Heavier Gases (e.g., Krypton, Xenon)
Heavier noble gases like Krypton (Kr) and Xenon (Xe) can provide a much higher sputtering yield than Argon because of their greater mass. However, they are substantially rarer and more expensive, limiting their use to highly specialized applications where maximizing the deposition rate is the absolute priority.
Reactive Gases (e.g., Nitrogen, Oxygen)
In a process called reactive sputtering, a secondary gas is intentionally introduced alongside the Argon. For example, adding nitrogen (N₂) gas would result in the deposition of an aluminum nitride (AlN) film, a ceramic, instead of a pure aluminum film. This is not used for pure Al deposition but is a common technique for creating compound thin films.
Making the Right Choice for Your Goal
Selecting the correct gas is fundamental to achieving the desired outcome in your deposition process.
- If your primary focus is cost-effective deposition of a pure aluminum film: Argon is the unquestionable industry standard and the correct choice.
- If your primary focus is achieving the maximum possible deposition rate, regardless of cost: Consider using a heavier, more expensive gas like Krypton or Xenon.
- If your primary focus is depositing a compound material like aluminum nitride (AlN): Use Argon as the primary sputtering gas and introduce Nitrogen as a secondary reactive gas.
Ultimately, choosing the right working gas is the first step in controlling the purity, quality, and efficiency of your sputtered film.
Summary Table:
| Gas Type | Role in Sputtering | Best For | Key Characteristics |
|---|---|---|---|
| Argon (Ar) | Primary Working Gas | Standard pure Al deposition | Inert, ideal atomic mass, cost-effective, stable plasma |
| Krypton/Xenon | High-Yield Alternative | Maximum deposition rate (specialized) | Heavier mass, higher sputtering yield, expensive |
| Nitrogen/Oxygen | Reactive Gas (with Ar) | Depositing compounds (e.g., AlN) | Chemically reacts with Al to form ceramic films |
Ready to optimize your sputtering process? The right equipment and consumables are key to achieving consistent, high-quality thin films. KINTEK specializes in providing high-purity lab equipment, including sputtering systems and gases, tailored to your laboratory's specific research and production goals.
Contact our experts today to discuss how we can support your thin film deposition projects with reliable solutions and expert guidance.
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Evaporation Boat for Organic Matter
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Electron Beam Evaporation Coating Oxygen-Free Copper Crucible and Evaporation Boat
- Aluminized Ceramic Evaporation Boat for Thin Film Deposition
People Also Ask
- Can carbon be sputtered? Unlock the Power of Diamond-Like Carbon (DLC) Films
- What are the primary uses of Ultra-Low Temperature (ULT) freezers in laboratories? Preserve Your Most Valuable Samples
- Does THC evaporate over time? The Truth About Potency Loss and Preservation
- What are the advantages and disadvantages of hot forming and cold forming? A Guide to Precision vs. Formability
- What is the function of a laboratory ultrasonic cleaner in the surface treatment workflow for pure titanium?
- What is the primary purpose of an oven in geopolymer pretreatment? Ensuring Moisture Stability and Process Precision
- How does carburizing work? Achieve Superior Surface Hardness and Core Toughness
- Which element made stainless steel difficult to brazed? It's Chromium's Oxide Layer



















