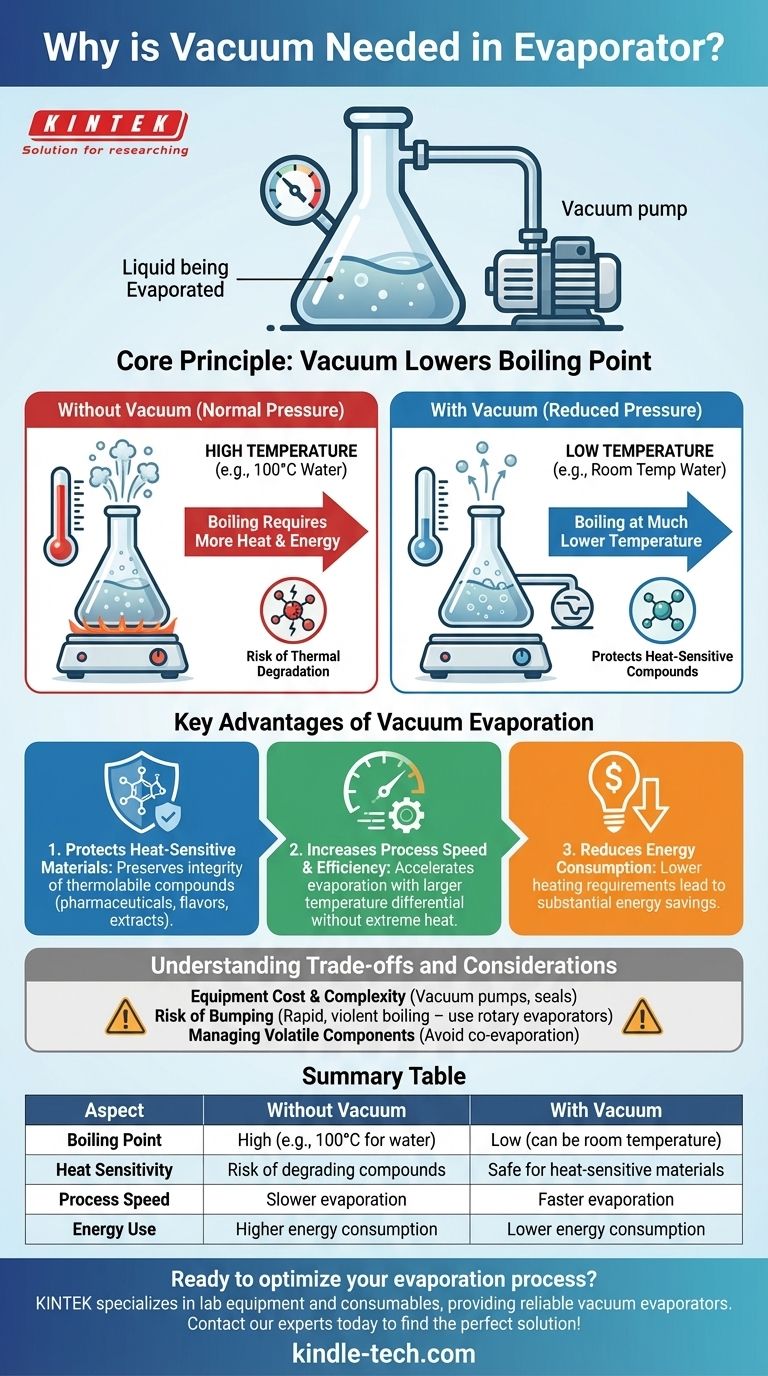
A vacuum is used in an evaporator to lower the boiling point of the liquid being evaporated. This fundamental principle allows for the rapid removal of a solvent at a much lower temperature than would be required under normal atmospheric pressure. This is crucial for preventing the thermal degradation of heat-sensitive compounds and increasing overall process efficiency.
The core reason for using a vacuum is to manipulate the physics of boiling. By reducing the ambient pressure, you make it significantly easier for a liquid to turn into a vapor, allowing for gentle, fast, and energy-efficient evaporation.

The Core Principle: How Pressure Controls Boiling Point
The entire process hinges on the relationship between a liquid's vapor pressure and the ambient pressure surrounding it. Understanding this interaction is key to understanding the purpose of the vacuum.
What Defines "Boiling"?
A liquid boils when its vapor pressure—the pressure exerted by its vapor molecules—equals the ambient pressure of the environment pressing down on its surface.
At sea level, water must be heated to 100°C (212°F) for its vapor pressure to match the surrounding atmospheric pressure and begin boiling.
How a Vacuum Changes the Equation
A vacuum pump actively removes air and other gases from the evaporator, drastically reducing the ambient pressure inside the system.
With less pressure pushing down on the liquid's surface, the liquid's vapor pressure does not need to climb as high to initiate boiling.
The Practical Result: Lower Temperature Evaporation
Because a lower vapor pressure is now sufficient for boiling, the liquid does not need to be heated to a high temperature.
For example, under a strong vacuum, water can be made to boil at room temperature. This effect allows you to precisely control the evaporation temperature by adjusting the level of the vacuum.
Key Advantages of Vacuum Evaporation
Applying this principle provides several critical operational benefits, making it an indispensable technique in both laboratory and industrial settings.
Protecting Heat-Sensitive Materials
This is the most critical advantage. Many valuable compounds in pharmaceuticals, food products (flavors and aromas), and natural extracts are thermally labile, meaning they are easily damaged or destroyed by heat.
Evaporating the solvent at a low temperature ensures the integrity and potency of the target compound is preserved.
Increasing Process Speed and Efficiency
Evaporation speed is driven by the temperature difference between the heating source and the liquid.
By lowering the liquid's boiling point, you can create a larger and more effective temperature differential without resorting to extreme heat. This accelerates the rate of heat transfer and speeds up the entire evaporation process.
Reducing Energy Consumption
Heating a substance to a lower temperature requires significantly less energy. In large-scale industrial applications, reducing the boiling point by even 20-30°C can translate into substantial energy savings and lower operational costs.
Understanding the Trade-offs and Considerations
While powerful, vacuum evaporation is not without its complexities. Acknowledging the trade-offs is essential for proper implementation.
Equipment Cost and Complexity
Implementing a vacuum requires specialized equipment, including vacuum pumps, controllers, and airtight seals on the evaporator. This adds a layer of cost and maintenance complexity compared to simple atmospheric boiling.
The Risk of "Bumping"
Under vacuum, boiling can sometimes be too rapid, causing violent bursts of vapor that splash the product out of the container. This phenomenon, known as bumping, can lead to sample loss.
Modern systems like rotary evaporators (rotovaps) mitigate this by rotating the flask to ensure smooth, even evaporation.
Managing Volatile Components
Careful control is necessary. If the vacuum is too strong or the temperature is too high, you risk co-evaporating not just the target solvent but also other volatile components of your desired product, leading to a loss of yield or quality.
Applying This to Your Goal
Choosing the right evaporation method depends entirely on the material you are working with and your primary objective.
- If your primary focus is preserving heat-sensitive compounds: A vacuum evaporator is non-negotiable to prevent product degradation.
- If your primary focus is industrial-scale speed and energy efficiency: Vacuum evaporation is the superior method for reducing operational costs and increasing throughput.
- If your primary focus is simply removing a heat-stable solvent from a non-volatile product (e.g., water from salt): Simple atmospheric boiling may be a more cost-effective solution.
Ultimately, using a vacuum gives you precise control over the evaporation process, safeguarding your product while maximizing efficiency.
Summary Table:
| Aspect | Without Vacuum | With Vacuum |
|---|---|---|
| Boiling Point | High (e.g., 100°C for water) | Low (can be room temperature) |
| Heat Sensitivity | Risk of degrading compounds | Safe for heat-sensitive materials |
| Process Speed | Slower evaporation | Faster evaporation |
| Energy Use | Higher energy consumption | Lower energy consumption |
Ready to optimize your evaporation process? KINTEK specializes in lab equipment and consumables, providing reliable vacuum evaporators that protect your valuable heat-sensitive samples while increasing your lab's efficiency. Contact our experts today to find the perfect solution for your laboratory's needs!
Visual Guide
Related Products
- Circulating Water Vacuum Pump for Laboratory and Industrial Use
- Molybdenum Tungsten Tantalum Evaporation Boat for High Temperature Applications
- Oil Free Diaphragm Vacuum Pump for Laboratory and Industrial Use
- Ceramic Evaporation Boat Set Alumina Crucible for Laboratory Use
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
People Also Ask
- What are the primary considerations for selecting Tungsten Carbide (WC) grinding balls? Optimize Your Milling Process
- What are the parts of a kiln sitter? A Guide to the Essential Safety Mechanism
- Why are glass balls and bottles used for milling lithium-carbon anodes? Optimize Material Safety and Purity
- How do you maintain a vacuum pump in a lab? Ensure Reliable Performance and Extend Lifespan
- What is the function of a magnetic stirrer in SiOC film preparation? Ensure Precision in Precursor Mixing
- What is the difference between crystalline and fused quartz? A Guide to Atomic Structure and Material Properties
- What are the different types of temperature sensors? Choose the Right Sensor for Your Application
- Why use zirconia grinding kits for LATP synthesis? Ensure High Purity and Ionic Conductivity



















