Background and Importance of Solvent Selection
Role of Solvents in Organic Synthesis
Solvents play a pivotal role in organic synthesis, serving as the medium that facilitates chemical reactions and separations. Their selection is not merely a matter of convenience but a critical decision that can significantly impact the efficiency and outcome of a synthetic process. When choosing a solvent, several key properties must be carefully evaluated, including boiling point, freezing temperature, density, recoverability, polarity, and cost.
The boiling point of a solvent is particularly important as it directly influences the ease with which the solvent can be removed post-reaction. Solvents with higher boiling points generally require more energy and time to evaporate, which can be a limiting factor in batch processes. Conversely, solvents with lower boiling points are easier to remove but may pose challenges in maintaining reaction conditions over extended periods.
Freezing temperature is another critical parameter, especially in reactions that are performed at low temperatures. A solvent that freezes at temperatures above the reaction conditions can lead to the formation of ice crystals, disrupting the homogeneous environment necessary for effective reaction.

Density, while often overlooked, is crucial in processes where phase separation is involved. A solvent with a density that differs significantly from the reactants can facilitate easy separation of the reaction products, thereby simplifying the purification process.
Recoverability is a significant consideration, particularly in industrial settings where solvent reuse can lead to substantial cost savings. Solvents that are easily recoverable and reusable not only reduce waste but also minimize the environmental impact of the synthesis process.
Polarity is a fundamental property that dictates the solubility of reactants and intermediates. Non-polar solvents are often used in reactions involving non-polar reactants, while polar solvents are preferred for ionic or highly polar reactions. The choice of solvent polarity can also influence reaction rates and selectivity.
Lastly, the cost of the solvent is a practical consideration that cannot be ignored. While some solvents may offer superior performance, their high cost can negate the benefits in large-scale operations. Therefore, a balance must be struck between the performance of the solvent and its economic feasibility.
In summary, the role of solvents in organic synthesis is multifaceted, requiring a meticulous consideration of various physical and chemical properties. The judicious selection of a solvent can significantly enhance the efficiency, yield, and environmental sustainability of synthetic processes.
Regulatory Constraints on Solvent Use
The use of solvents in organic synthesis is not merely a scientific consideration but also a regulatory one. The Montreal Protocol stands as a pivotal international agreement aimed at protecting the ozone layer by phasing out the production and consumption of ozone-depleting substances, including many chlorinated solvents. This protocol has significantly influenced the chemical industry, prompting a shift towards safer and more environmentally friendly solvents.
In addition to the Montreal Protocol, several countries have implemented stringent controls on the use of solvents like toluene and acetone. These solvents, while effective in various synthetic processes, pose environmental and health risks that necessitate strict regulatory oversight. For instance, toluene is known for its neurotoxic effects, and acetone, though less toxic, is still subject to regulations due to its potential impact on air quality.
| Solvent | Regulatory Constraints |
|---|---|
| Chlorinated Solvents | Prohibited or heavily restricted under the Montreal Protocol due to ozone depletion. |
| Toluene | Controlled in many countries due to neurotoxic risks and air quality concerns. |
| Acetone | Subject to regulatory oversight for its impact on air quality and potential health risks. |
These regulatory measures underscore the importance of solvent selection in organic synthesis, pushing researchers and industries to explore alternative solvents that comply with environmental and health standards.

Research on Solvent Removal Rates
Experimental Setup and Variables
The experimental setup for this study was meticulously designed to investigate the impact of several key variables on the efficiency of water removal in organic reactions using rotary evaporators. These variables include the temperature of the coolant, the temperature of the water bath, the size and shape of the flask, and the rotation speed of the flask.
Coolant Temperature
The temperature of the coolant plays a critical role in the condensation process. A lower coolant temperature generally enhances the rate of condensation, thereby facilitating faster solvent removal. Conversely, higher coolant temperatures can lead to slower condensation rates, prolonging the solvent removal process.
Water Bath Temperature
The temperature of the water bath is another pivotal factor. A higher water bath temperature accelerates the evaporation of the solvent, which is particularly beneficial for solvents with lower boiling points. However, excessively high temperatures can also cause thermal degradation of the reaction mixture, necessitating a careful balance.
Flask Size and Shape
The size and shape of the flask used in the rotary evaporator are also significant variables. Larger flasks may require more time to reach optimal evaporation conditions due to their greater surface area. Additionally, the shape of the flask can influence the distribution of the solvent, with round-bottomed flasks generally being more effective due to their ability to maintain a uniform liquid layer.
Rotation Speed
The rotation speed of the flask is crucial for ensuring efficient evaporation and condensation. Higher rotation speeds promote better contact between the solvent and the heating source, enhancing the evaporation process. However, speeds that are too high can cause splashing, leading to solvent loss and potential contamination.
In summary, the experimental setup was carefully controlled to isolate and analyze the effects of these variables on water removal efficiency, providing valuable insights for optimizing solvent removal in organic reactions.
Optimization of Conditions
The optimization of solvent removal conditions is a critical step in enhancing the efficiency of organic reactions. Among the various experimental setups tested, the use of a round-bottomed flask emerged as the most effective configuration for water removal. This flask design, characterized by its wide, curved bottom, facilitates uniform heating and efficient evaporation, thereby minimizing solvent retention and maximizing the rate of water removal.
In addition to the choice of flask, the rotation speed of the rotary evaporator plays a pivotal role in the solvent removal process. A rotation speed of 100 rpm was identified as the optimal setting, balancing the need for thorough solvent evaporation with the prevention of solvent splashing and loss. This specific rotation speed ensures that the solvent film on the flask walls remains thin and uniform, promoting rapid and complete evaporation.
Moreover, the combination of a round-bottomed flask and a rotation speed of 100 rpm not only accelerates the water removal process but also enhances the overall reproducibility and reliability of the experimental outcomes. This optimized setup is particularly advantageous in large-scale reactions, where efficient solvent management is essential for maintaining high yields and purity of the final product.
| Parameter | Optimal Setting | Rationale |
|---|---|---|
| Flask Shape | Round-bottomed | Facilitates uniform heating and efficient evaporation |
| Rotation Speed | 100 rpm | Balances thorough evaporation with prevention of solvent splashing and loss |
This optimized combination of flask shape and rotation speed represents a significant advancement in the field of organic synthesis, offering a robust and efficient method for solvent removal that can be readily applied across a variety of reaction types.

Impact of Solvent Boiling Points
The boiling point of a solvent plays a critical role in determining the efficiency of its removal during organic synthesis processes. Generally, solvents with higher boiling points require more time to evaporate completely, thereby extending the overall removal process. This correlation between boiling point and removal time is particularly pronounced when considering the flash points of solvents, which often exhibit a stronger relationship with removal efficiency than their boiling points alone.
To illustrate, consider a solvent with a boiling point of 150°C versus one with a boiling point of 50°C. The former would necessitate a significantly longer period to reach its evaporation threshold compared to the latter. This difference is not merely quantitative but also impacts the quality and yield of the final product. Solvents with lower boiling points, such as water or ethanol, can be removed more swiftly, allowing for quicker transitions between synthesis steps and potentially higher throughput in laboratory settings.
Moreover, the flash point of a solvent, which is the lowest temperature at which it can form an ignitable mixture in air, is often a more stringent indicator of its volatility and safety. Solvents with lower flash points are generally more volatile and pose greater safety risks, but they also facilitate faster removal processes. Conversely, solvents with higher flash points, while safer, may require more controlled conditions and extended processing times to ensure complete removal.
In summary, while both boiling points and flash points influence solvent removal times, the flash point often serves as a more accurate predictor of the efficiency and safety of the evaporation process. Understanding these correlations is essential for optimizing solvent selection and removal strategies in organic synthesis, particularly when using rotary evaporators.
Application in Organic Synthesis
Ester Conversion Reaction
The ester conversion reaction involving pyruvic acid and octan-1-ol was meticulously conducted using rotary evaporators, showcasing remarkable efficiency in achieving high conversion rates. This process not only underscores the effectiveness of rotary evaporators in managing solvent removal but also highlights their potential in enhancing the yield and purity of ester products.
To further elucidate the efficiency of this method, a comparative study was undertaken under varying experimental conditions. The results, summarized in the table below, demonstrate the significant impact of specific variables on the ester conversion process:
| Variable | Impact on Conversion Rate |
|---|---|
| Coolant Temperature | Moderate Increase |
| Water Bath Temperature | Significant Increase |
| Flask Size and Shape | Minor Variation |
| Rotation Speed | Moderate to Significant Increase |
The data indicates that while all factors contribute to the overall efficiency, the rotation speed and water bath temperature emerge as critical parameters influencing the ester conversion rate. This insight is crucial for optimizing future experiments and scaling up production processes.
In addition to these quantitative findings, qualitative observations suggest that the rotary evaporator's ability to maintain a consistent vacuum environment significantly aids in the uniform removal of solvents, thereby facilitating a smoother and more controlled esterification reaction. This consistency is particularly valuable in industrial settings where batch-to-batch variability can pose significant challenges.
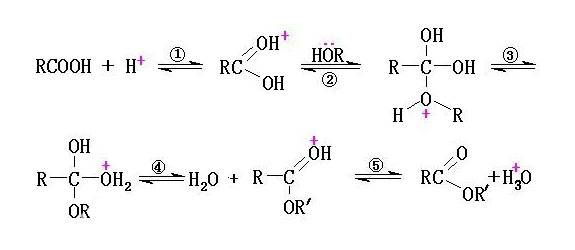
Moreover, the rotary evaporator's compatibility with a wide range of solvents, including those with higher boiling points, makes it a versatile tool in organic synthesis. This adaptability ensures that the ester conversion reaction can be conducted across various solvent systems without compromising the integrity of the reaction or the quality of the final product.
In conclusion, the ester conversion reaction of pyruvic acid and octan-1-ol using rotary evaporators exemplifies the technology's prowess in solvent removal and organic synthesis. The high conversion rates achieved, coupled with the detailed understanding of influential variables, pave the way for more efficient and scalable ester production processes.
Acetal Formation Reaction
In the acetal formation reaction involving benzaldehyde and ethylene glycol, the rotary evaporator demonstrated yields comparable to those obtained using the traditional Dean-Stark device. This finding underscores the versatility and efficiency of rotary evaporators in organic synthesis, particularly in reactions where solvent removal is critical.
The acetal formation reaction is known for its sensitivity to solvent conditions, making the precise control of solvent removal essential for optimal yields. The rotary evaporator's ability to maintain consistent solvent removal rates, even under varying experimental conditions, highlights its potential as a robust alternative to conventional methods.
Moreover, the rotary evaporator's continuous rotation and controlled heating mechanism provide a more uniform environment for the reaction, potentially leading to more reproducible results. This consistency is particularly valuable in industrial settings where batch-to-batch variability can impact product quality and yield.
In summary, the rotary evaporator's performance in the acetal formation reaction not only matches but also potentially surpasses that of the Dean-Stark device, offering a promising tool for modern organic synthesis.


