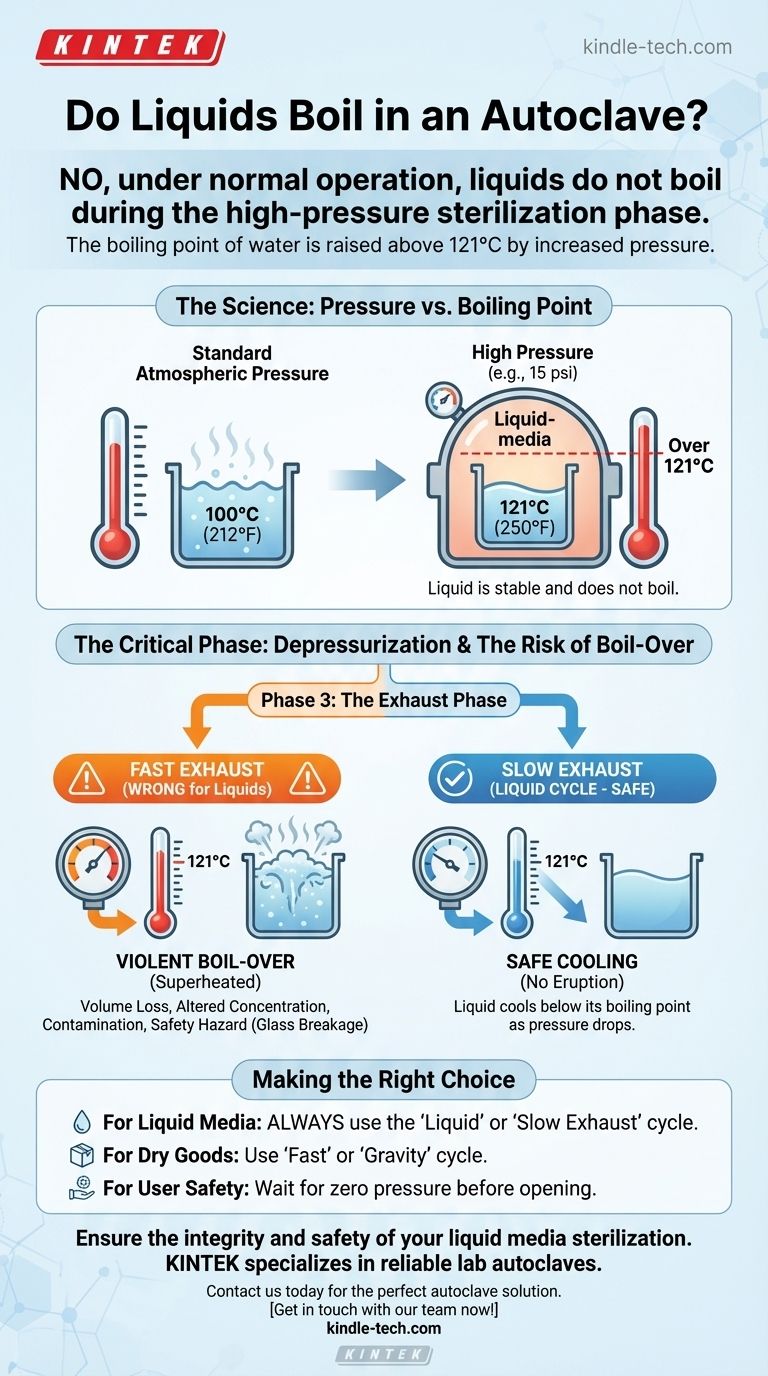
No, under normal operation, liquids do not boil during the high-pressure sterilization phase of an autoclave cycle. This is by design. However, they can boil over violently and dangerously during the depressurization phase if the incorrect settings are used, which is the primary concern when sterilizing liquids.
The core principle is that an autoclave increases pressure to raise the boiling point of water well above the sterilization temperature of 121°C. The real risk of boiling occurs after sterilization, when a rapid drop in pressure can cause the still-hot liquid to erupt.

The Physics of Sterilization: Pressure vs. Boiling Point
To understand why liquids behave this way, you must first understand the fundamental principle of an autoclave. It is not simply an oven; it is a high-pressure vessel.
How an Autoclave Works
An autoclave is essentially a sophisticated pressure cooker. It uses high-pressure steam to achieve temperatures capable of killing microorganisms, spores, and viruses.
Elevating the Boiling Point
At standard sea-level atmospheric pressure, water boils at 100°C (212°F). By increasing the pressure inside the chamber to approximately 15 psi (pounds per square inch) above atmospheric pressure, an autoclave forces the boiling point of water to increase to 121°C (250°F).
The State of the Liquid During Sterilization
During the sterilization phase, your liquid media is held at 121°C. While this is far above its normal boiling point, it is below the boiling point at that elevated pressure. The liquid is therefore thermodynamically stable and does not boil.
Deconstructing the Autoclave Cycle for Liquids
The risk of boiling is entirely concentrated in one specific part of the cycle. Understanding these phases is critical for safe operation.
Phase 1: The Purge and Heat-Up
The cycle begins by removing air from the chamber and replacing it with steam. Both pressure and temperature rise together until they reach the target for sterilization (e.g., 15 psi and 121°C).
Phase 2: The Sterilization Dwell
This is the main sterilization period, where the chamber is held at a constant high temperature and pressure for a set time (typically 15-20 minutes). As explained above, no boiling occurs here.
Phase 3: The Critical Exhaust Phase
After the dwell time is complete, the pressure must be released. This is the most dangerous part of the process for liquids. If the pressure is vented too quickly, the boiling point of your liquid instantly drops back down to 100°C.
Since your liquid is still at 121°C, it is now suddenly superheated and will boil instantaneously and violently. This is known as boil-over.
Understanding the Trade-offs and Dangers
Choosing the wrong autoclave cycle is the most common mistake and has significant consequences. The central trade-off is speed versus safety.
The Primary Danger: Boil-Over
A boil-over event is not just a minor inconvenience. It leads to several problems:
- Loss of Volume: You lose a significant portion of your carefully prepared media.
- Altered Concentration: The loss of water vapor changes the concentration of salts and nutrients in the remaining media.
- Contamination: The liquid can bubble up and wet the container's filter or plug, creating a pathway for contaminants to enter as it cools.
- Safety Hazard: In extreme cases, the rapid boiling can cause glass containers to crack or shatter, creating a significant safety risk.
The Critical Choice: Fast vs. Slow Exhaust
Autoclaves have different cycles for different materials.
- Fast Exhaust (Gravity/Dry Cycle): This cycle vents pressure quickly. It is designed for dry goods like glassware, instruments, and waste. It is not safe for liquids.
- Slow Exhaust (Liquid Cycle): This cycle releases pressure very slowly, allowing the liquid to cool down in tandem with the dropping pressure. This keeps the liquid's temperature below the ever-changing boiling point, preventing boil-over.
Making the Right Choice for Your Goal
To ensure safety and effectiveness, always match the autoclave cycle to the material you are sterilizing.
- If your primary focus is sterilizing liquid media (e.g., broth, agar): Always use the designated "liquid" or "slow exhaust" cycle to prevent boil-over and ensure the integrity of your media.
- If your primary focus is speed and throughput: Never use a fast or "gravity" cycle for liquids; reserve these cycles exclusively for dry goods like glassware or instruments.
- If your primary focus is user safety: Allow the autoclave to complete its full slow exhaust cycle and wait for the chamber pressure to return to zero before attempting to open the door.
Understanding this pressure-temperature relationship is the key to achieving safe, repeatable, and effective liquid sterilization.
Summary Table:
| Phase | Pressure | Temperature | Boiling Risk | Key Action |
|---|---|---|---|---|
| Sterilization Dwell | High (e.g., 15 psi) | High (121°C) | None | Liquid is stable below its elevated boiling point. |
| Fast Exhaust (Wrong for Liquids) | Drops rapidly | Stays at 121°C | High - Boil-Over | Liquid becomes superheated and erupts. |
| Slow Exhaust (Liquid Cycle) | Drops slowly | Cools gradually | None | Temperature stays below boiling point, preventing eruption. |
Ensure the integrity and safety of your liquid media sterilization.
Choosing the right autoclave cycle is critical for preventing dangerous boil-overs and maintaining media concentration. KINTEK specializes in providing reliable lab autoclaves and expert support for all your laboratory sterilization needs.
Contact us today to find the perfect autoclave solution for your lab. Let our experts help you achieve safe, repeatable results.
Get in touch with our team now!
Visual Guide
Related Products
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Portable Digital Display Automatic Laboratory Sterilizer Lab Autoclave for Sterilization Pressure
- Desktop Fast Laboratory Autoclave Sterilizer 20L 24L for Lab Use
- Portable High Pressure Laboratory Autoclave Steam Sterilizer for Lab Use
- Laboratory Sterilizer Lab Autoclave Pulsating Vacuum Desktop Steam Sterilizer
People Also Ask
- Why is a high-pressure steam autoclave necessary for dilute acid pretreatment? Unlock Biomass Potential at 120°C
- Why is autoclaving the best method for sterilization? Achieve 100% Sterility with Pressurized Steam
- What is a laboratory autoclave? A Guide to Sterilization with Pressurized Steam
- What medical equipment can be autoclaved? Ensure Sterile, Safe, and Durable Instruments
- What is the size of a laboratory autoclave? A Guide to Choosing the Right Capacity
- What can you use instead of autoclave? Find the Right Sterilization Method for Your Materials
- What role does a high-pressure stainless steel autoclave play in synthesizing FOTS-TiO2? Mastering Material Morphology
- What are the advantages of autoclaving in hospitals? Achieve Unmatched Sterilization for Patient Safety



















