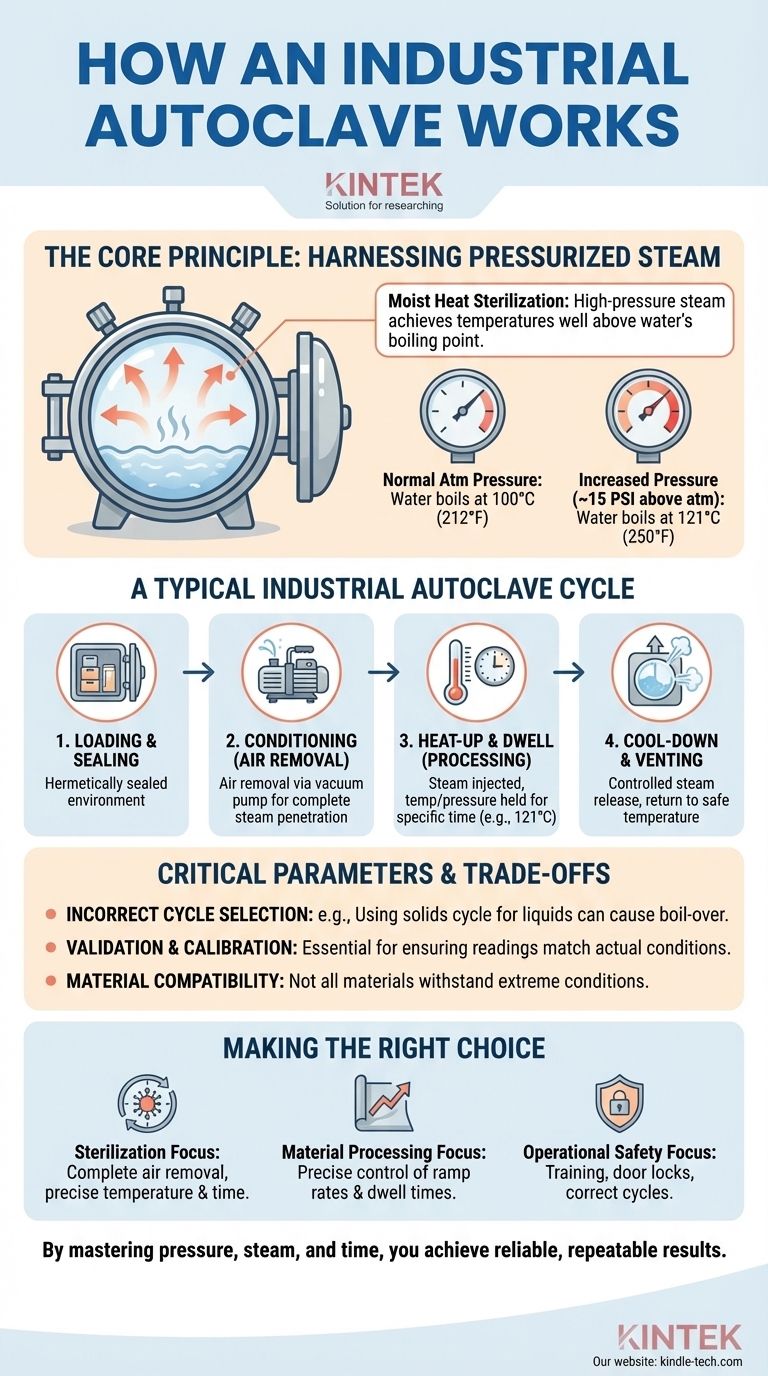
At its core, an industrial autoclave works by using high-pressure steam to achieve temperatures well above the normal boiling point of water. This process, known as moist heat sterilization or processing, leverages the direct relationship between pressure and temperature. By creating a sealed, high-pressure environment, the autoclave can efficiently transfer immense thermal energy into materials, enabling rapid sterilization or fundamental changes in the material's physical properties.
An industrial autoclave is not simply a heater. It is a precision-engineered pressure vessel that harnesses the physics of steam to create conditions impossible at normal atmospheric pressure, enabling deep, rapid, and reliable material processing.

The Core Principle: Harnessing Pressurized Steam
To truly understand an autoclave, you must move beyond the idea of just "heat." The magic is in the combination of heat, pressure, and water in its vapor state (steam).
Why Steam, Not Just Hot Air?
Moist heat is significantly more effective at transferring energy than dry heat. Steam condenses on the surface of the item being processed, rapidly transferring its thermal energy and penetrating porous materials far more effectively than hot, dry air.
This is why moist heat sterilization can achieve in 15-20 minutes what might take hours for a dry heat oven at a much higher temperature.
The Physics of Pressure and Temperature
Under normal atmospheric pressure, water boils at 100°C (212°F). No matter how much heat you apply, it will not get hotter.
An autoclave is a hermetically sealed chamber. As water inside is heated, it turns to steam, and since the steam cannot escape, the pressure inside the chamber rises.
This increased pressure raises the boiling point of water. For example, at roughly 15 PSI (pounds per square inch) above atmospheric pressure, the boiling point of water rises to 121°C. This allows the autoclave to achieve the high temperatures necessary for sterilization or material curing.
A Typical Industrial Autoclave Cycle
While specific cycles vary by application (e.g., medical sterilization vs. composite curing), the fundamental stages are consistent.
Stage 1: Loading and Sealing
Items are loaded into the chamber, ensuring there is adequate space for steam to circulate. The heavy, robust door is then closed and locked, creating a hermetically sealed environment. This seal is critical for building and maintaining pressure.
Stage 2: Conditioning (Air Removal)
This is one of the most critical stages. Any air trapped within the chamber can create insulating "cold spots" where steam cannot reach, leading to incomplete processing or sterilization failure.
Industrial autoclaves use methods like a vacuum pump to remove nearly all the air before introducing steam, ensuring complete steam penetration.
Stage 3: Heat-Up and Dwell (Processing)
Steam is injected into the vacuumed chamber, or a reservoir of water within is boiled to generate steam. The system rapidly reaches the target temperature and pressure (e.g., 121°C or 134°C).
The autoclave then "dwells" or holds at this setpoint for a prescribed amount of time, which is determined by the load size and material type. This is the primary processing or sterilization phase.
Stage 4: Cool-Down and Venting
Once the dwell time is complete, the steam is vented from the chamber in a controlled manner, causing the pressure to drop back to atmospheric levels. The load is also allowed to cool to a safe temperature before the door can be unlocked.
Understanding the Trade-offs and Critical Parameters
An autoclave is a powerful tool, but its effectiveness depends entirely on precise control. Misunderstanding its operation can lead to failed processes or safety hazards.
The Risk of Incorrect Cycle Selection
Choosing the wrong program is a common failure point. For example, using a standard "solids" cycle for liquids can cause them to boil over violently during the rapid depressurization phase. A dedicated "liquids" cycle uses a much slower, controlled cooling and venting process to prevent this.
The Importance of Validation and Calibration
You cannot simply trust the number on the screen. Calibration is the process of using external temperature and pressure probes to verify that the autoclave's display readings match the actual conditions inside the chamber.
Validation is a more comprehensive process that proves a specific cycle consistently sterilizes or processes a specific load, every single time. For regulated industries like medical device manufacturing or aerospace, this is non-negotiable.
Material Compatibility
Not all materials can withstand the extreme conditions inside an autoclave. Certain plastics may melt, and sensitive electronics will be destroyed. Always confirm that the materials being processed are rated for the temperatures and pressures of your selected cycle.
Making the Right Choice for Your Goal
Understanding these principles allows you to use the autoclave as a predictable engineering tool rather than a black box.
- If your primary focus is sterilization and quality assurance: Your priority must be validating that your cycles achieve complete air removal and maintain the correct temperature for the required duration.
- If your primary focus is material processing (e.g., composites, rubber): Your focus should be on the precise control of temperature and pressure ramp rates and dwell times, as these directly influence the final material properties.
- If your primary focus is operational safety: Your priorities are rigorous operator training, ensuring door-locking mechanisms are functioning, and using correct cycles to prevent hazards like liquid boil-over.
By mastering the interplay of pressure, steam, and time, you transform the autoclave from a simple machine into a tool for achieving reliable and repeatable results.
Summary Table:
| Stage | Key Action | Purpose |
|---|---|---|
| 1. Loading & Sealing | Items loaded; door sealed shut. | Creates a hermetically sealed chamber to build pressure. |
| 2. Conditioning | Air is removed via vacuum pump. | Eliminates cold spots for complete steam penetration. |
| 3. Heat-Up & Dwell | Steam injected; temperature/pressure held. | Primary sterilization or material processing phase. |
| 4. Cool-Down & Venting | Controlled release of steam and pressure. | Safely returns the chamber to ambient conditions. |
Ready to achieve reliable, high-temperature sterilization and material processing?
KINTEK specializes in high-performance industrial autoclaves and lab equipment, designed for precision, safety, and compliance in demanding environments. Whether your focus is medical device sterilization, composite curing, or advanced material research, our solutions deliver the validated results you need.
Contact our experts today to discuss your specific requirements and discover how KINTEK can enhance your laboratory's capabilities.
Visual Guide
Related Products
- Laboratory High Pressure Steam Sterilizer Vertical Autoclave for Lab Department
- Portable Digital Display Automatic Laboratory Sterilizer Lab Autoclave for Sterilization Pressure
- Portable High Pressure Laboratory Autoclave Steam Sterilizer for Lab Use
- Laboratory High Pressure Horizontal Autoclave Steam Sterilizer for Lab Use
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
People Also Ask
- What is the necessity of using an autoclave for pre-treating culture media? Ensure Accurate Ag2O/TiO2 Testing
- What role does an autoclave play in the acid treatment for microalgae disruption? Unlock High-Yield Cell Pretreatment
- What critical environmental conditions does a laboratory autoclave provide for evaluating wear resistance? - KINTEK
- Why is the standard autoclave temperature set to 121? The Science of Effective Sterilization
- What is the primary function and principle of autoclaving? Master Lab Sterilization with High-Pressure Steam



















