To properly check and maintain an autoclave, you must implement a multi-layered program of daily operational checks, routine cleaning, and periodic performance validation. This involves inspecting physical components like door gaskets, cleaning the chamber to prevent residue buildup, and regularly using biological and chemical indicators to confirm that the machine is achieving complete sterilization.
The core principle of autoclave maintenance is not just about keeping the machine running, but about systematically verifying its ability to achieve sterility. A truly effective program combines routine user upkeep with scheduled professional validation to ensure consistent, reliable, and safe operation.

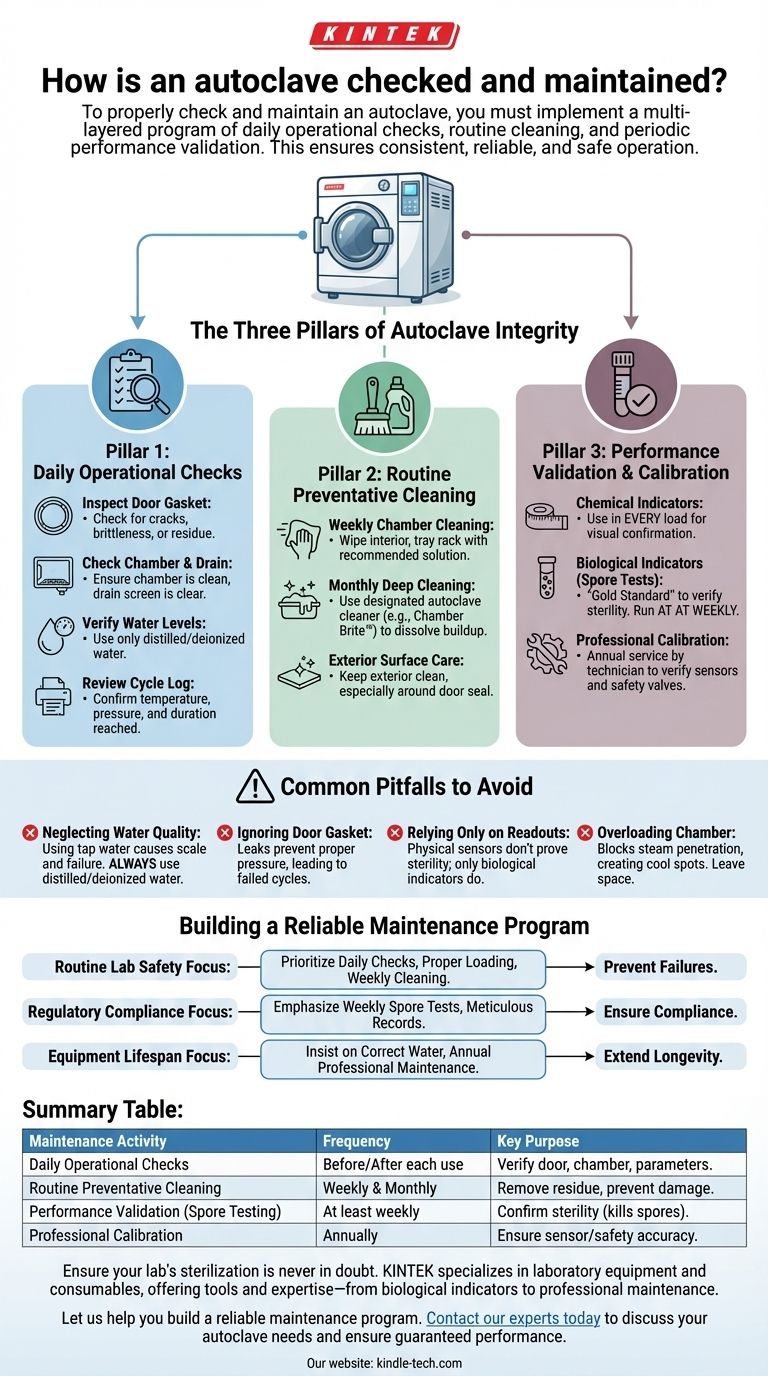
The Three Pillars of Autoclave Integrity
An effective maintenance strategy is built on three distinct but interconnected types of activities. Each one addresses a different aspect of the autoclave's performance and longevity.
Pillar 1: Daily Operational Checks
These are simple but critical inspections performed by the operator before and after each use. They are the first line of defense against cycle failures.
Inspect the Door Gasket: The gasket creates the pressure-tight seal. Look for any signs of cracking, brittleness, or residue that could prevent a proper seal.
Check the Chamber and Drain Screen: Before loading, ensure the chamber is free of debris from previous cycles. Check that the chamber's drain screen is clear to allow for proper steam circulation and drying.
Verify Water Levels (If Applicable): For autoclaves with an integrated steam generator, ensure the reservoir has the correct amount of distilled or deionized water. Using tap water can cause mineral buildup and damage internal components.
Review the Cycle Printout/Log: After a cycle, always check the physical or digital record. Confirm that the cycle reached the programmed temperature and pressure and held them for the required duration.
Pillar 2: Routine Preventative Cleaning
Regular cleaning prevents the buildup of residue and mineral scale, which can interfere with steam generation, clog valves, and damage the chamber itself.
Weekly Chamber Cleaning: Wipe down the interior of the chamber, tray rack, and trays with a recommended cleaning solution or distilled water. This removes any spilled media or residue.
Monthly Deep Cleaning: Perform a more thorough cleaning of the chamber using a designated autoclave cleaning solution (like Chamber Brite™ or a manufacturer-approved equivalent). This process dissolves mineral deposits and other buildup that regular wiping cannot remove.
Exterior Surface Care: Keep the exterior of the unit clean. Pay special attention to the area around the door seal to ensure no debris gets trapped.
Pillar 3: Performance Validation and Calibration
While daily checks and cleaning ensure the machine runs, validation proves it sterilizes. This is a non-negotiable step in any clinical, pharmaceutical, or research setting.
Chemical Indicators: These are strips or tapes that change color when exposed to specific temperatures. A chemical indicator should be placed inside every load to provide a quick visual confirmation that the load was exposed to sterilization conditions.
Biological Indicators (Spore Tests): This is the gold standard for verifying sterility. A biological indicator contains highly resistant bacterial spores (like Geobacillus stearothermophilus). After a cycle, the vial is incubated to see if any spores survived.
Spore Testing Frequency: The accepted best practice is to run a spore test at least weekly. It should also be performed after any major repairs or whenever a new type of packaging material is introduced.
Professional Calibration: At least annually, a qualified technician should perform a full preventative maintenance and calibration service. They will verify temperature and pressure sensors against certified instruments, check safety valves, and replace worn components like gaskets and filters.
Common Pitfalls to Avoid
Even with a schedule, certain oversights can compromise sterilization and damage the equipment. Understanding these common failure points is critical.
Neglecting Water Quality
Using tap water or water with a high mineral content is one of the fastest ways to destroy an autoclave. The resulting scale buildup clogs pipes, coats heating elements, and can cause catastrophic component failure. Always use distilled or deionized water as specified by the manufacturer.
Ignoring the Door Gasket
The door gasket is the most common point of failure. A leaking gasket prevents the chamber from reaching the required pressure, leading to an immediate cycle abort or, worse, an incomplete sterilization cycle that appears successful.
Relying Only on Physical Readouts
The autoclave's screen or printout only shows that the sensors reached a certain temperature and pressure. It does not prove that steam penetrated the load effectively or that microorganisms were killed. Only a biological indicator (spore test) provides definitive proof of sterilization.
Overloading the Chamber
Placing items too close together or stacking them improperly prevents steam from penetrating all surfaces. This creates cool spots within the load where microorganisms can survive. Always leave adequate space between items for steam circulation.
Building a Reliable Maintenance Program
Your approach should be tailored to your specific needs for compliance, safety, and equipment longevity.
- If your primary focus is routine lab safety: Prioritize daily operational checks, proper loading techniques, and weekly chamber cleaning to prevent cycle failures and contamination.
- If your primary focus is regulatory compliance (e.g., medical or pharma): Emphasize rigorous weekly biological indicator testing and meticulous record-keeping for every cycle and maintenance activity.
- If your primary focus is extending equipment lifespan: Insist on using the correct water quality and adhering to a strict schedule of annual professional calibration and preventative maintenance.
Ultimately, consistent and well-documented maintenance is the only way to transform your autoclave from an appliance into a reliable instrument for guaranteed sterilization.
Summary Table:
| Maintenance Activity | Frequency | Key Purpose |
|---|---|---|
| Daily Operational Checks | Before/After each use | Verify door seal, chamber condition, and cycle parameters. |
| Routine Preventative Cleaning | Weekly & Monthly | Remove residue and mineral scale to prevent damage. |
| Performance Validation (Spore Testing) | At least weekly | Confirm sterility by killing resistant bacterial spores. |
| Professional Calibration | Annually | Ensure sensors and safety systems are accurate. |
Ensure your lab's sterilization is never in doubt. Proper autoclave maintenance is critical for safety, compliance, and protecting your investment. KINTEK specializes in laboratory equipment and consumables, offering the tools and expertise you need—from biological indicators for validation to professional maintenance services.
Let us help you build a reliable maintenance program. Contact our experts today to discuss your autoclave needs and ensure guaranteed performance.
Visual Guide
Related Products
- Portable High Pressure Laboratory Autoclave Steam Sterilizer for Lab Use
- Laboratory Sterilizer Lab Autoclave Herbal Powder Sterilization Machine for Plant
- Laboratory Sterilizer Lab Autoclave Pulsating Vacuum Desktop Steam Sterilizer
- Laboratory High Pressure Steam Sterilizer Vertical Autoclave for Lab Department
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
People Also Ask
- When loading the instruments into the autoclave should you label it? Ensure Safe, Traceable Sterilization Every Time
- What are the 3 stages of autoclave sterilization? Master the Purge, Exposure & Exhaust Phases
- What can you use instead of autoclave? Find the Right Sterilization Method for Your Materials
- What is the temperature of a low autoclave? The Critical Minimum for Sterilization
- Why is it important to use the autoclave to sterilize laboratory tools? Ensure Complete Sterility for Reliable Results
- Why is autoclave better than dry-heat? Achieve Faster, More Efficient Sterilization
- What are the different types of autoclaves in microbiology? Gravity vs. Pre-Vacuum Explained
- Is it necessary to have an autoclave? Ensure True Sterility for Your Lab or Clinic



















