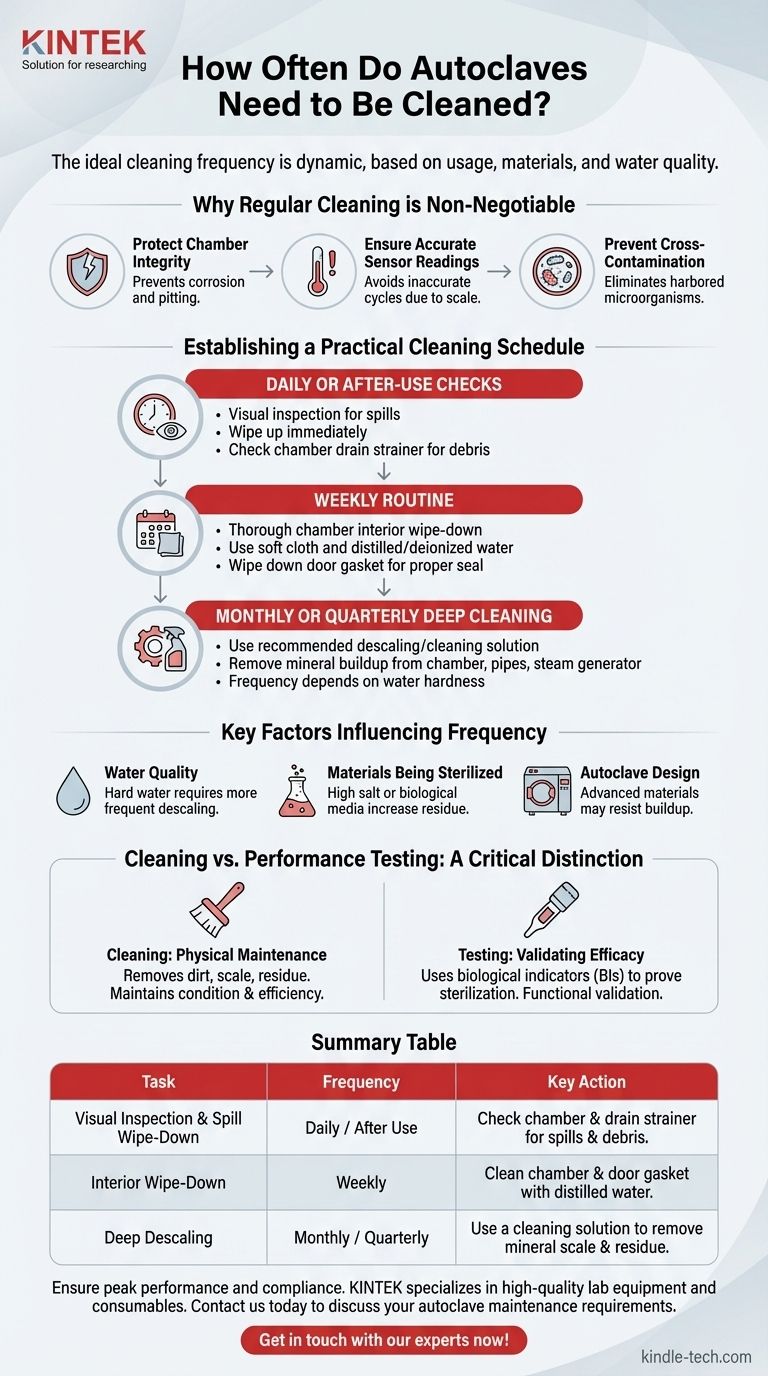
The ideal cleaning frequency for an autoclave is not a single, fixed schedule but a dynamic protocol based on usage, the materials being sterilized, and your water quality. For most labs, a routine of daily visual checks, weekly wipe-downs, and a monthly or quarterly deep clean serves as an effective starting point.
The core issue is not simply cleaning for appearance, but preventing the buildup of mineral scale and residue. This buildup can damage the autoclave's chamber and sensors, interfere with heat distribution, and ultimately compromise the effectiveness of every sterilization cycle.

Why Regular Cleaning is Non-Negotiable
An autoclave is a precision instrument. Its performance relies on the clean, efficient transfer of heat and steam. Neglecting regular cleaning directly undermines this core function.
Protecting Chamber Integrity
Residue from sterilized media or dissolved minerals from hard water can bake onto the chamber walls. Over time, these deposits can cause pitting and corrosion, permanently damaging the stainless steel and shortening the equipment's lifespan.
Ensuring Accurate Sensor Readings
Modern autoclaves rely on sensitive temperature and pressure sensors to control the sterilization cycle. A buildup of residue or scale can insulate these sensors, leading to inaccurate readings and potentially incomplete sterilization.
Preventing Cross-Contamination
Spilled biological media or other residues can become baked-on deposits. These can harbor microorganisms or chemical contaminants that may aerosolize in subsequent cycles, leading to cross-contamination of otherwise clean loads.
Establishing a Practical Cleaning Schedule
Instead of a one-size-fits-all rule, a tiered approach based on frequency of use is most effective. Always consult your specific model's user manual as the definitive guide.
Daily or After-Use Checks
A simple visual inspection is your first line of defense. Check for and immediately wipe up any spills inside the chamber. You should also check the chamber drain strainer for debris and clean it as needed to prevent clogs.
Weekly Routine
Perform a more thorough cleaning of the chamber interior using a soft cloth and distilled or deionized water. Wipe down the door gasket to remove any residue, ensuring a proper seal can be formed.
Monthly or Quarterly Deep Cleaning
This involves using a recommended descaling or autoclave cleaning solution to remove mineral buildup from the chamber, pipes, and steam generator. The frequency of this step is highly dependent on the hardness of your water supply.
Cleaning vs. Performance Testing: A Critical Distinction
It is crucial to understand that physical cleaning is different from performance validation testing. While both are essential for proper autoclave management, they serve separate purposes.
The Goal of Cleaning: Physical Maintenance
Cleaning, as described above, is the physical removal of dirt, scale, and residue. Its purpose is to maintain the equipment's physical condition, ensure efficient operation, and prevent damage.
The Goal of Testing: Validating Efficacy
Performance testing uses biological indicators (BIs) to prove that the autoclave is achieving the required temperature and pressure for the necessary time to kill highly resistant microorganisms. This is a functional validation, not a physical one.
Integrating Both into Your Protocol
Regulatory guidelines often mandate a testing schedule. For example, autoclaves inactivating human pathogens may require testing after every 40 hours of use, while others might be tested monthly or semi-annually. Your cleaning schedule should support this testing; a clean autoclave is far more likely to pass its validation tests.
Key Factors That Influence Cleaning Frequency
Your specific protocol should be adjusted based on several environmental and usage factors.
Water Quality
This is the single most significant factor. Facilities using hard tap water will experience rapid mineral scale buildup and require frequent, aggressive descaling. Using distilled or deionized water can dramatically reduce this need.
Materials Being Sterilized
Loads with high salt concentrations or biological growth media (like agar) are more likely to boil over or leave residue. Frequent sterilization of these materials necessitates a more frequent cleaning schedule compared to sterilizing clean glassware.
Autoclave Design
Some modern autoclaves feature advanced chamber materials or coatings designed to resist buildup and make cleaning easier. Refer to the manufacturer's documentation to understand your unit's specific characteristics.
Creating Your Autoclave Maintenance Protocol
Use these guidelines to build a protocol that matches your lab's specific needs. Always prioritize your manufacturer's recommendations and your facility's internal Standard Operating Procedures (SOPs).
- If your primary focus is high-throughput use with biological media: Institute a weekly deep clean with a descaling agent and perform daily visual checks and spill removal.
- If your primary focus is sterilizing clean glassware or instruments: A monthly deep clean combined with weekly wipe-downs is likely a sufficient and effective schedule.
- If your primary focus is compliance in a clinical or regulated environment: Strictly adhere to the validated SOPs for your facility, which will specify both cleaning and performance testing frequencies.
A proactive and consistent maintenance routine is the most reliable way to ensure effective sterilization and protect your investment.
Summary Table:
| Task | Frequency | Key Action |
|---|---|---|
| Visual Inspection & Spill Wipe-Down | Daily / After Use | Check chamber and drain strainer for spills and debris. |
| Interior Wipe-Down | Weekly | Clean chamber and door gasket with distilled water. |
| Deep Descaling | Monthly / Quarterly | Use a cleaning solution to remove mineral scale and residue. |
Ensure your autoclave operates at peak performance and compliance. Proper maintenance is critical for reliable sterilization and protecting your lab equipment investment. KINTEK specializes in high-quality lab equipment and consumables, serving all your laboratory needs. Contact us today to discuss your autoclave maintenance requirements or to explore our range of reliable laboratory solutions. Get in touch with our experts now!
Visual Guide
Related Products
- Portable High Pressure Laboratory Autoclave Steam Sterilizer for Lab Use
- Laboratory Sterilizer Lab Autoclave Pulsating Vacuum Desktop Steam Sterilizer
- Laboratory High Pressure Steam Sterilizer Vertical Autoclave for Lab Department
- Laboratory Sterilizer Lab Autoclave Herbal Powder Sterilization Machine for Plant
- Portable Digital Display Automatic Laboratory Sterilizer Lab Autoclave for Sterilization Pressure
People Also Ask
- What core environmental conditions does a supercritical water autoclave provide? Simulating SCWR alloy performance.
- Why is an autoclave used for the Hydrogen Disbonding Test? Ensuring 5Cr-0.5Mo Steel Cladding Integrity
- How long should an autoclave last? Maximize Your Investment with Proper Care
- Why is a high-pressure steam sterilizer or autoclave required during the biomass pretreatment process? Optimize Yields
- Can autoclave sterilize liquid? Master Safe and Effective Liquid Sterilization
- Why is it important to use the autoclave to sterilize laboratory tools? Ensure Complete Sterility for Reliable Results
- What to avoid when using an autoclave? Prevent Common and Dangerous Sterilization Errors
- Why is the prevention of air entrapment critical for the autoclave sterilization process? Ensure 100% Sterility Today



















