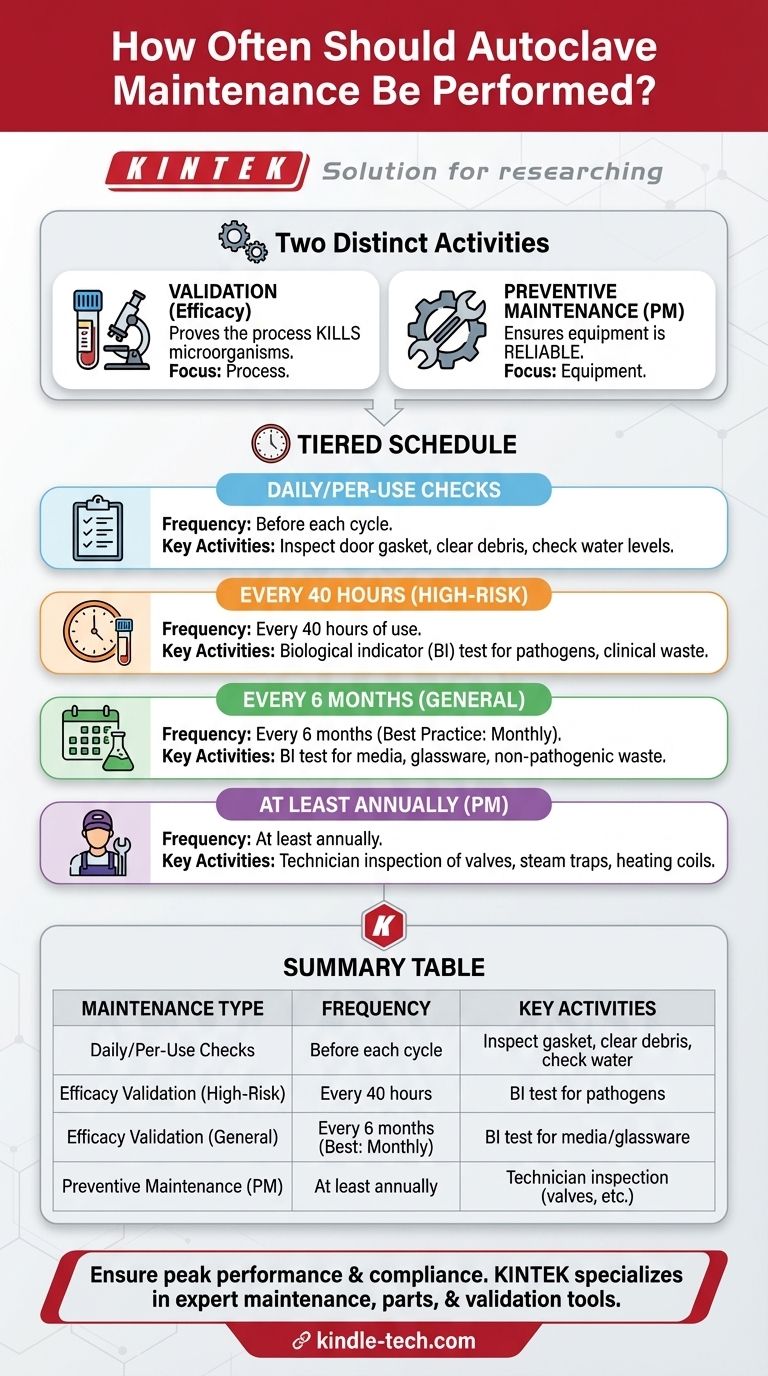
In short, autoclave maintenance is not a single event but a tiered schedule. Its frequency depends on the task and the materials being sterilized, ranging from per-use checks to routine validation every 40 hours for high-risk materials, and comprehensive hardware inspections performed at least annually by a qualified technician.
The core principle to understand is that "maintenance" consists of two distinct but equally critical activities: validation, which proves the autoclave can kill microorganisms, and preventive maintenance, which ensures the machine itself is functioning safely and reliably. Adhering to only one of these is a common and costly mistake.

The Layers of Autoclave Maintenance
True autoclave reliability is built on a multi-frequency schedule. Each layer addresses a different aspect of performance and safety, from daily operational checks to deep mechanical inspections.
Daily or Per-Use Operator Checks
Before running a cycle, the operator should perform basic checks. This is the first line of defense against immediate cycle failure or safety hazards.
These tasks include visually inspecting the door gasket for cracks, ensuring the chamber and drain screen are free of debris, and confirming there is sufficient water for steam generation.
Routine Efficacy Validation (Testing)
This is the most critical step for ensuring sterilization is actually occurring. It involves using biological indicators (BIs) to prove the cycle is lethal to highly resistant microorganisms.
The frequency of this validation depends directly on what you are sterilizing.
The 40-Hour Rule for High-Risk Materials
For autoclaves used to inactivate materials like human pathogens, blood, tissues, or clinical samples, validation with a biological indicator is required after every 40 hours of use.
This high frequency is a safety mandate to ensure that potentially infectious agents are consistently and verifiably destroyed.
The Six-Month Rule for General Materials
For autoclaves sterilizing lower-risk materials such as media, glassware, or non-pathogenic waste, a full validation test is typically required every six months.
However, many institutions adopt a best practice of running a BI test at least monthly to catch any performance drift before it becomes a major failure.
Scheduled Preventive Maintenance (PM)
This is a comprehensive inspection of the autoclave's mechanical and electrical components. It is not about testing efficacy; it is about ensuring the machine's long-term health and preventing unexpected downtime.
This service, performed by a qualified technician, should occur on a monthly, quarterly, or annual basis. An annual inspection is the absolute minimum standard.
During a PM visit, a technician will inspect, clean, and test parts like safety valves, steam traps, heating coils, and electronic contactors. This service is crucial for preventing damage from poor water quality or wear and tear.
Understanding the Trade-offs: Validation vs. Maintenance
Confusing efficacy validation with mechanical maintenance is a primary source of autoclave failure. They are not interchangeable.
Validation Confirms the Process Works
Using a biological indicator proves that the combination of time, temperature, and steam pressure in a cycle is sufficient to achieve sterilization. It validates the process.
A successful BI test tells you that on that day, with that load, the autoclave performed its primary function correctly.
Maintenance Ensures the Equipment is Reliable
Inspecting gaskets, cleaning steam traps, and testing safety valves ensures the machine can reliably and safely create the conditions for sterilization over hundreds of cycles. It maintains the equipment.
Neglecting this can lead to aborted cycles, steam leaks, or a catastrophic failure, even if the last BI test passed.
Making the Right Choice for Your Goal
To build a robust maintenance plan, you must layer these activities based on your specific application and risk tolerance.
- If your primary focus is biosafety with pathogens or clinical waste: You must validate with a biological indicator at least every 40 hours of use and perform annual preventive maintenance.
- If your primary focus is sterilizing general lab media or glassware: You should validate with a biological indicator monthly as a best practice, with a mandatory re-validation every six months.
- If your primary focus is maximizing uptime and equipment lifespan: You must implement a program of annual (or semi-annual) preventive maintenance with a technician, in addition to the required validation schedule.
A proactive, multi-layered maintenance plan is the only way to guarantee both consistent sterilization and long-term equipment reliability.
Summary Table:
| Maintenance Type | Frequency | Key Activities |
|---|---|---|
| Daily/Per-Use Checks | Before each cycle | Inspect door gasket, clear debris, check water levels |
| Efficacy Validation (High-Risk) | Every 40 hours of use | Biological indicator (BI) test for pathogens, clinical waste |
| Efficacy Validation (General) | Every 6 months (best practice: monthly) | BI test for media, glassware, non-pathogenic waste |
| Preventive Maintenance (PM) | At least annually | Technician inspection of valves, steam traps, heating coils |
Ensure your lab's autoclave operates at peak performance and compliance. KINTEK specializes in lab equipment and consumables, providing expert maintenance services, genuine parts, and validation tools to keep your sterilization process reliable and safe. Don't risk downtime or biosafety failures—contact our specialists today to schedule a service or consultation!
Visual Guide
Related Products
- Laboratory High Pressure Steam Sterilizer Vertical Autoclave for Lab Department
- Laboratory Sterilizer Lab Autoclave Herbal Powder Sterilization Machine for Plant
- Portable Digital Display Automatic Laboratory Sterilizer Lab Autoclave for Sterilization Pressure
- Portable High Pressure Laboratory Autoclave Steam Sterilizer for Lab Use
- Desktop Fast Laboratory Autoclave Sterilizer 20L 24L for Lab Use
People Also Ask
- What is the primary function of a laboratory pressure steam sterilizer in dark fermentation? Boost Hydrogen Yield
- What is the safety wall in an autoclave? The Jacketed Chamber Explained for Secure Sterilization
- What is the main purpose of the autoclave? Achieve Complete Sterilization with High-Pressure Steam
- Why is an Autoclave essential for simulating nuclear reactor conditions during the corrosion testing of zirconium alloys?
- Is an autoclave a medical device? Understanding Regulatory Classification and Intended Use
- Is it necessary to have an autoclave? Ensure True Sterility for Your Lab or Clinic
- What is the temperature of autoclave in microbiology lab? Achieve Sterile Conditions with 121°C
- Why use Zirconia rods for sample mounting in high-pressure autoclaves? Ensure Data Purity and Chemical Stability.