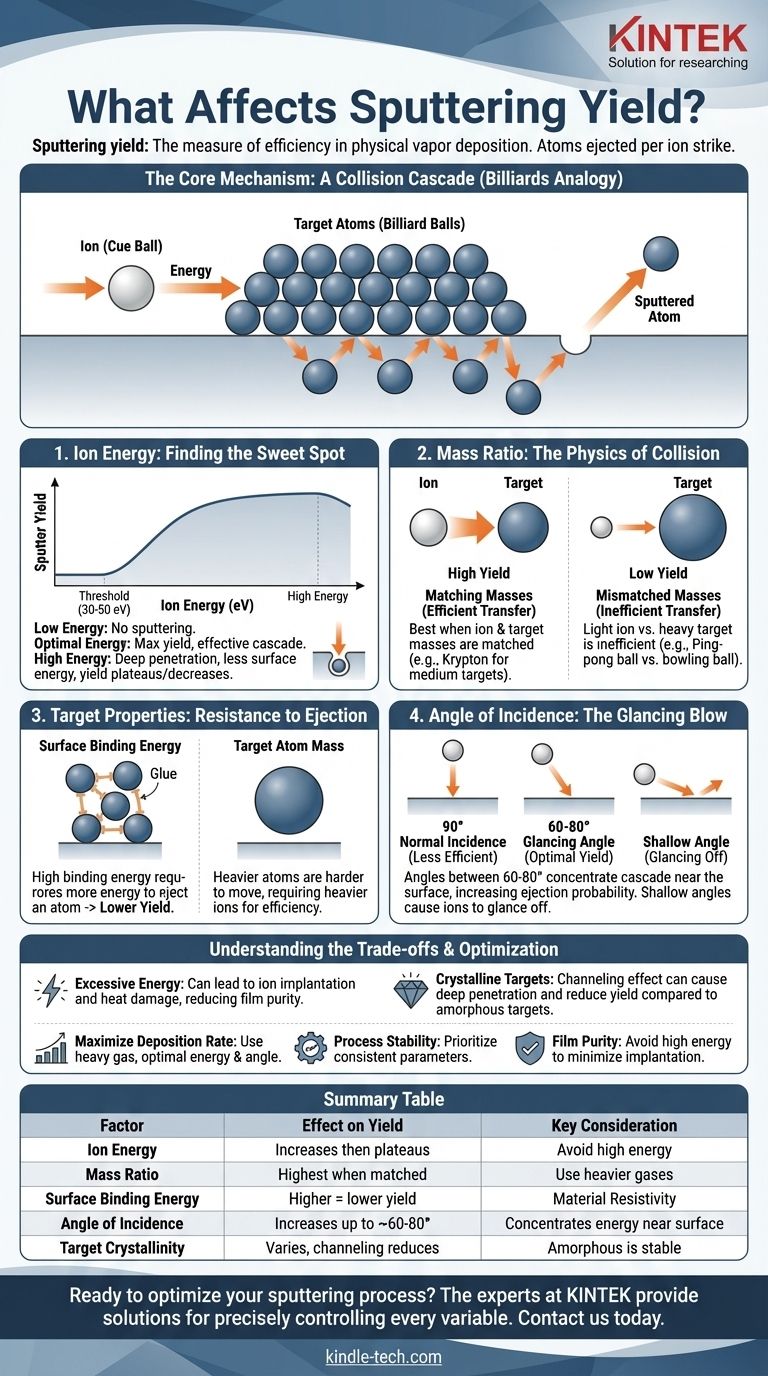
Sputtering yield is the single most important measure of efficiency in a physical vapor deposition process. At its core, the yield is the average number of atoms ejected from a target material for each individual ion that strikes it. This efficiency is governed by a precise interplay between the energy and mass of the incoming ion, the properties of the target material, and the geometry of the collision.
Sputtering is not a simple erosion process; it is a physical phenomenon driven by momentum transfer. The key to understanding and controlling sputter yield is to visualize how energy from an incoming particle is distributed within the first few atomic layers of a target to cause an atom to be ejected.

The Core Mechanism: A Collision Cascade
Think of sputtering as a subatomic game of billiards. An incoming ion—typically an inert gas like Argon—is the "cue ball," accelerated toward a rack of "billiard balls," which are the atoms of your target material.
When the ion strikes the target, it sets off a chain reaction, a collision cascade, under the surface. The goal is not for the initial ion to directly knock out a surface atom. Instead, the ion transfers its momentum to atoms within the target, which in turn collide with their neighbors. An atom is sputtered only when this cascade of energy works its way back to the surface and gives a surface atom enough energy to overcome its bonds and escape into the vacuum.
Key Factors Influencing Sputter Yield
The efficiency of this entire process depends on several critical, controllable variables.
Ion Energy: Finding the Sweet Spot
There is a minimum energy, or sputtering threshold, required to dislodge an atom, typically between 30 and 50 electron volts (eV). Below this, no sputtering occurs.
As ion energy increases above this threshold, the sputter yield rises because more energy is available to create a larger, more effective collision cascade.
However, this trend does not continue indefinitely. At very high energies (e.g., thousands of eV), the incoming ion penetrates too deeply into the target. The resulting collision cascade is centered far below the surface, and less of its energy is directed back toward the surface atoms, causing the yield to plateau or even decrease.
Mass Ratio: The Physics of Collision
The efficiency of momentum transfer depends heavily on the relative masses of the ion (cue ball) and the target atoms (billiard balls).
Maximum energy transfer occurs when the masses are closely matched. For example, using a heavier sputtering gas like Krypton instead of Argon to sputter a medium-mass target will generally increase the sputter yield. Using a very light ion to sputter a very heavy target (like hitting a bowling ball with a ping-pong ball) is highly inefficient.
Target Properties: Resistance to Ejection
The target material itself presents two primary barriers to sputtering.
Surface Binding Energy
This is the "glue" that holds the target atoms together. Materials with a high surface binding energy require more energy to liberate an atom, resulting in a lower sputter yield.
Target Atom Mass
Heavier target atoms are inherently more difficult to move. This ties back to the mass ratio; a heavier target requires a correspondingly heavier ion for efficient momentum transfer.
Angle of Incidence: The Glancing Blow
A direct, 90-degree impact is often not the most effective angle for sputtering.
Angling the ion bombardment (typically between 60-80 degrees from normal) concentrates the collision cascade nearer the surface. This increases the probability that the transferred energy will result in a surface atom being ejected, thus increasing the sputter yield. At very shallow angles, however, the ions are more likely to simply glance off the surface.
Understanding the Trade-offs
Simply maximizing the sputter yield is not always the best strategy, as it can introduce undesirable side effects.
The Problem with Excessive Energy
Pushing ion energy too high to chase a higher yield can lead to ion implantation, where the sputtering gas ions become embedded in the target and, subsequently, in your deposited film. This can contaminate the film and alter its properties. High energies also generate more heat, which can damage sensitive targets or substrates.
Crystalline vs. Amorphous Targets
For crystalline targets, the sputter yield can be highly dependent on the crystal orientation relative to the ion beam. Ions can travel down "channels" between atomic planes, penetrating deeply and reducing the sputter yield. This channeling effect can cause process instability if the target's crystal texture changes over time.
Optimizing Sputter Yield for Your Goal
Controlling these factors allows you to tailor the sputtering process to your specific objective.
- If your primary focus is maximizing deposition rate: Use a heavy sputtering gas (e.g., Krypton or Xenon) and operate at an ion energy and angle that corresponds to the peak of the yield curve for your specific target material.
- If your primary focus is process stability and repeatability: Prioritize maintaining a consistent ion energy, gas pressure, and angle. Be mindful that for crystalline targets, yield may shift as the target erodes and exposes new crystal facets.
- If your primary focus is film purity and quality: Avoid excessively high ion energies to minimize ion implantation and heat-related damage, even if it results in a lower deposition rate.
Ultimately, mastering sputter yield is about controlling the physics of collision to achieve your specific material deposition goals.
Summary Table:
| Factor | Effect on Sputter Yield | Key Consideration |
|---|---|---|
| Ion Energy | Increases up to a plateau, then decreases | Avoid high energies to prevent ion implantation |
| Ion/Target Mass Ratio | Highest yield when masses are matched | Use heavier gases (Kr, Xe) for heavy targets |
| Target Surface Binding Energy | Higher energy = lower yield | Material property that resists sputtering |
| Angle of Incidence | Increases up to ~60-80°, then decreases | Glancing angles concentrate energy near surface |
| Target Crystallinity | Varies with orientation; amorphous is stable | Channeling effect in crystals can reduce yield |
Ready to optimize your sputtering process for maximum yield and superior film quality? The experts at KINTEK are here to help. We specialize in providing the right lab equipment and consumables to precisely control every variable—from ion source selection to target material properties.
Contact us today to discuss how our solutions can enhance your deposition efficiency, improve process stability, and achieve your specific material science goals.
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Electron Beam Evaporation Coating Oxygen-Free Copper Crucible and Evaporation Boat
- VHP Sterilization Equipment Hydrogen Peroxide H2O2 Space Sterilizer
People Also Ask
- What are the advantages of plasma enhanced CVD? Enable Low-Temperature, High-Quality Thin Film Deposition
- What is plasma activated chemical vapor deposition? Enable Low-Temperature Thin Film Deposition
- How does plasma vapor deposition work? A Low-Temperature Coating Solution for Sensitive Materials
- What is plasma enhanced? A Guide to Low-Temperature, High-Precision Manufacturing
- What is the plasma CVD process? Achieve Low-Temperature Thin Film Deposition



















