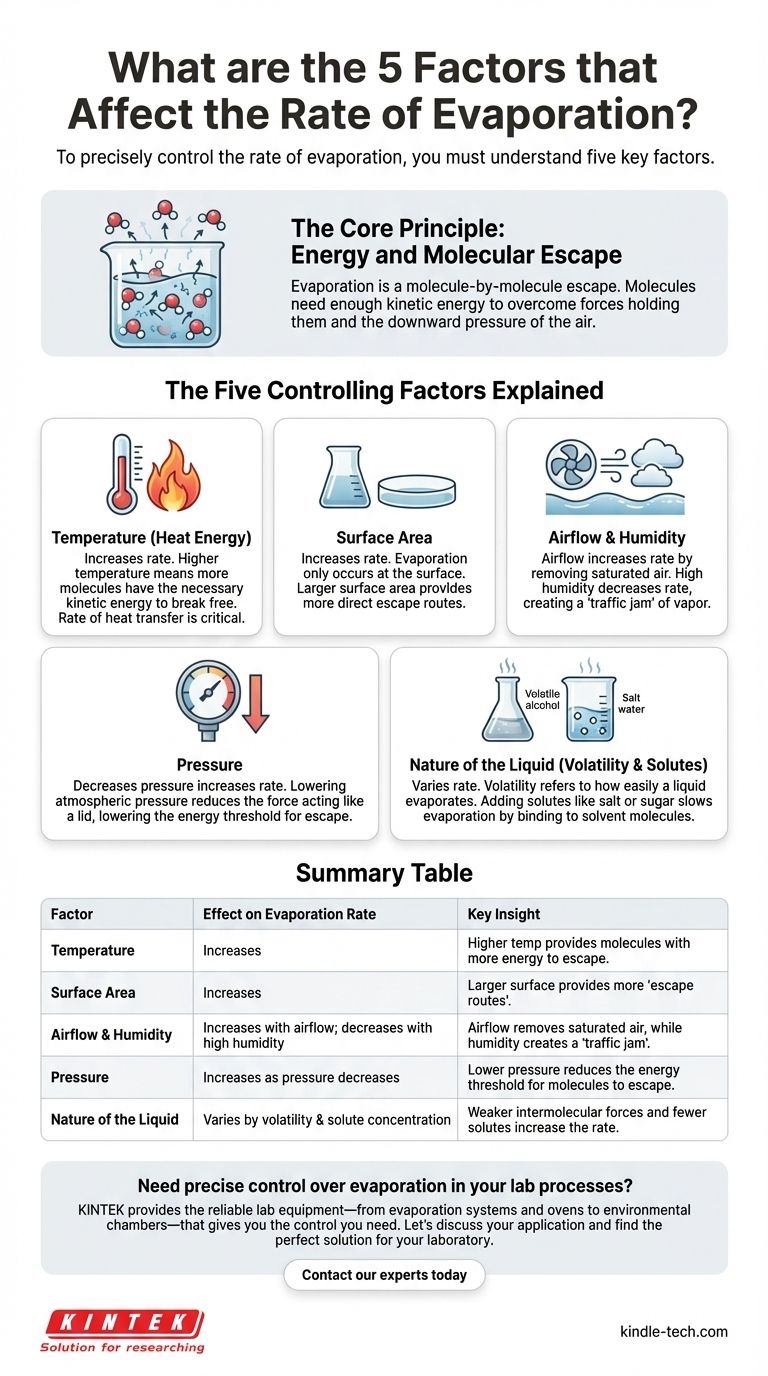
To precisely control the rate of evaporation, you must understand five key factors that govern it. These are the temperature of the liquid, the amount of exposed surface area, the movement and humidity of the air above the surface, the ambient pressure, and the inherent properties of the liquid itself. Mastering these variables allows you to either accelerate or suppress the process to meet a specific goal.
Evaporation is fundamentally a process of energy transfer. Its speed is determined by the balance between the energy available to liquid molecules and the physical barriers—like air pressure and intermolecular forces—that prevent their escape into a gaseous state.

The Core Principle: Energy and Molecular Escape
Evaporation might seem like a passive process, but it's an active, molecule-by-molecule escape. Understanding this core mechanism is key to manipulating its rate.
Molecules in Constant Motion
The molecules within a liquid are in constant, chaotic motion. Their speed, and therefore their kinetic energy, is directly related to the liquid's temperature.
The Escape Threshold
For a molecule to evaporate, it must have enough kinetic energy to overcome both the forces holding it to its neighboring molecules and the downward pressure of the air above it. Only the fastest-moving molecules at the surface can achieve this and transition into a gas.
The Five Controlling Factors Explained
Each of the five factors directly influences either the energy of the molecules or the difficulty of their escape.
1. Temperature (Heat Energy)
Temperature is the most significant driver of evaporation. A higher temperature means more molecules have the necessary kinetic energy to break free from the liquid's surface.
The rate of heat transfer is critical. The faster you can supply energy to the liquid, the more consistently you can maintain a high rate of evaporation.
2. Surface Area
Evaporation only occurs at the surface of a liquid. By increasing the surface area, you increase the number of molecules that have a direct path to escape.
Think of a spilled glass of water: it evaporates far faster as a wide, thin puddle than it would if left in the tall, narrow glass. More surface means more escape routes.
3. Airflow and Humidity
The air directly above the liquid's surface can become saturated with vapor, creating a "traffic jam" that prevents more molecules from escaping.
Airflow (wind) sweeps this saturated air away, replacing it with drier air and clearing the path for further evaporation. Conversely, high humidity means the air is already near saturation, drastically slowing the process.
4. Pressure
The air in the atmosphere exerts a physical pressure on the surface of a liquid, acting like a lid.
Lowering the atmospheric pressure reduces the force pushing down on the surface. This lowers the energy threshold required for molecules to escape, thereby increasing the evaporation rate. This is why water boils at a lower temperature at high altitudes.
5. Nature of the Liquid (Volatility & Solutes)
Not all liquids are created equal. A liquid's inherent properties determine the baseline energy required for its molecules to escape.
Volatility refers to how easily a liquid evaporates. Liquids with weaker intermolecular forces, like alcohol or gasoline, are more volatile and evaporate much faster than water.
Adding solutes (like salt or sugar to water) makes it harder for the solvent molecules to escape, slowing evaporation. The solute particles effectively get in the way and bind to the water molecules, increasing the energy needed for them to break free.
Understanding the Trade-offs and Interconnections
These factors rarely act in isolation. Understanding how they interact is crucial for predicting outcomes in real-world scenarios.
Temperature vs. Humidity
Heating the air around a liquid does two things simultaneously: it transfers energy to the liquid, and it lowers the air's relative humidity. Both effects work together to dramatically accelerate evaporation.
Boiling is Not Evaporation
While related, these are distinct processes. Evaporation is a surface phenomenon that can occur at any temperature. Boiling is a rapid, bulk phenomenon that occurs throughout the liquid when its internal vapor pressure equals the external atmospheric pressure.
The Impact of Solutes Over Time
In industrial processes like food drying, as water evaporates, the concentration of solutes (sugars, salts) increases. This steadily raises the energy requirement for the remaining water to evaporate, causing the drying rate to slow down over time.
How to Apply This to Your Goal
Your strategy for controlling evaporation depends entirely on your desired outcome.
- If your primary focus is to maximize the rate of evaporation (e.g., drying clothes, industrial dehydration): Focus on increasing temperature, maximizing airflow across the surface, and spreading the liquid to expand its surface area.
- If your primary focus is to minimize the rate of evaporation (e.g., preserving a reservoir, storing chemicals): Focus on covering the surface to reduce area and block airflow, lowering the temperature, and protecting the system from wind.
- If you are working with a complex mixture: You must account for the liquid's inherent volatility and the changing concentration of any dissolved solutes, which will alter the energy requirements throughout the process.
By understanding these five fundamental factors, you can move from simply observing evaporation to actively controlling it for any application.
Summary Table:
| Factor | Effect on Evaporation Rate | Key Insight |
|---|---|---|
| Temperature | Increases | Higher temperature provides more molecules with the energy to escape. |
| Surface Area | Increases | A larger surface provides more 'escape routes' for molecules. |
| Airflow & Humidity | Increases with airflow; decreases with high humidity | Airflow removes saturated air, while humidity creates a 'traffic jam'. |
| Pressure | Increases as pressure decreases | Lower pressure reduces the energy threshold for molecules to escape. |
| Nature of the Liquid | Varies by volatility and solute concentration | Weaker intermolecular forces and fewer solutes increase the rate. |
Need precise control over evaporation in your lab processes? Whether you're developing new materials, concentrating samples, or conducting essential research, understanding and manipulating these factors is critical. KINTEK provides the reliable lab equipment—from evaporation systems and ovens to environmental chambers—that gives you the control you need. Let's discuss your application and find the perfect solution for your laboratory. Contact our experts today to get started.
Visual Guide
Related Products
- Laboratory Vertical Water Circulating Vacuum Pump for Lab Use
- Evaporation Crucible for Organic Matter
- Evaporation Boat for Organic Matter
- Wall Mounted Water Distillation Unit
People Also Ask
- What is the difference between thermal evaporator and e-beam evaporator? Choose the Right PVD Method for Your Thin Films
- What are the disadvantages of thermal evaporation method? Key Limitations in Purity and Performance
- What is the difference between e-beam evaporation and thermal evaporation? A Guide to Choosing the Right PVD Method
- What materials are used in evaporation? Selecting the Right Metals & Compounds for Thin Films
- How does an electron beam evaporator work? Achieve High-Purity Thin Film Deposition
- What is e-beam evaporation? Achieve High-Purity Thin Film Deposition for Your Lab
- How does temperature affect the rate of evaporation? Master the Science for Better Lab Results
- What is a thermal evaporation system? A Guide to Simple, Effective Thin-Film Deposition



