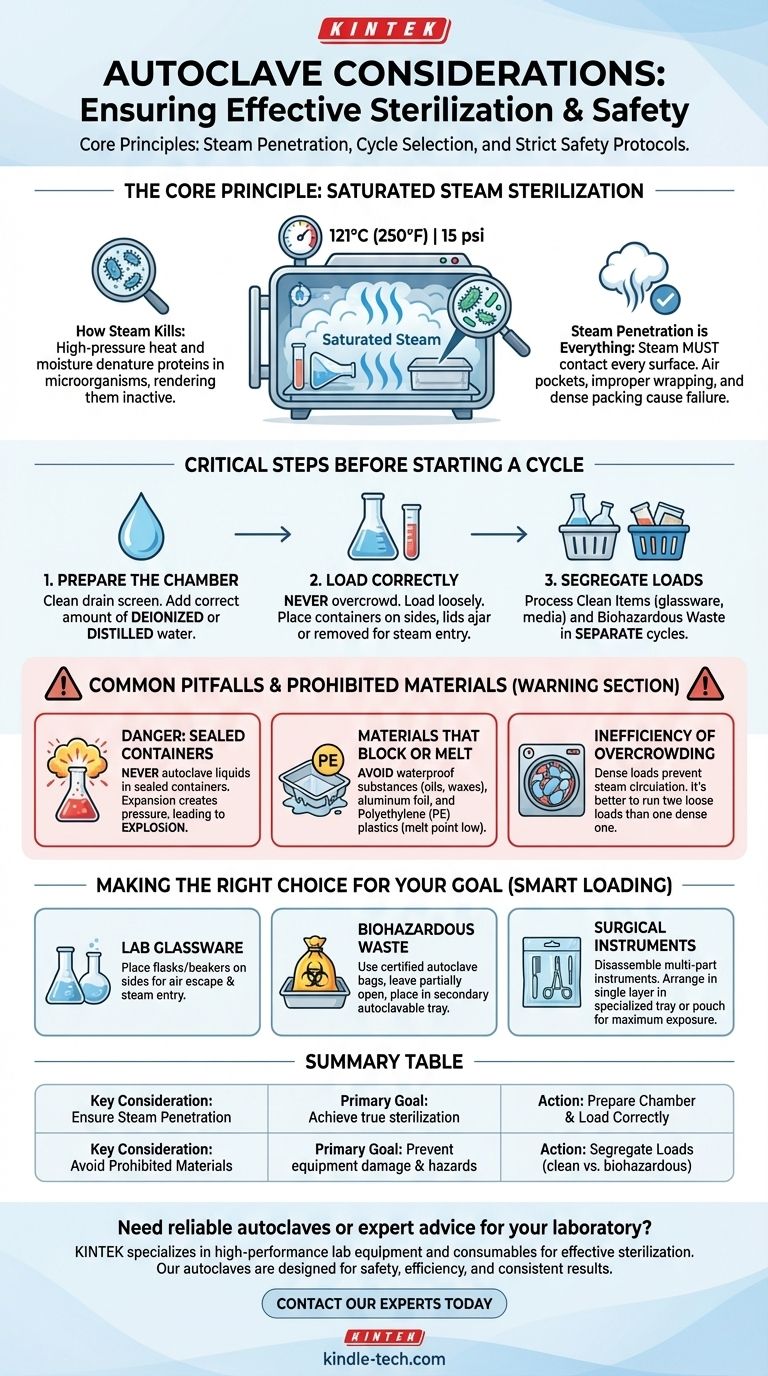
The most critical considerations for using an autoclave are ensuring proper steam penetration, selecting the correct cycle for the load, and adhering to strict safety protocols. This involves correctly preparing the chamber with water, loading materials without overcrowding, and never autoclaving prohibited items like sealed containers or waterproof substances.
The effectiveness of an autoclave does not come from heat alone, but from the complete penetration of high-pressure saturated steam. Every consideration, from how you load items to what materials you exclude, is designed to ensure steam can contact every surface to achieve true sterilization.

The Core Principle: Saturated Steam Sterilization
An autoclave is more than just a pressure cooker; it is a precision instrument designed for a specific purpose. Understanding its core mechanism is the key to using it effectively and safely.
How Steam Kills Microorganisms
An autoclave creates an environment of high-pressure saturated steam, typically reaching 121°C (250°F) at 15 psi. This combination of heat and moisture is highly effective at denaturing the proteins in microorganisms like bacteria, viruses, and spores, rendering them inactive and non-infectious.
Why Steam Penetration is Everything
The entire process fails if steam cannot reach every surface of the items being sterilized. Air pockets, improper wrapping, or dense packing create barriers that insulate microbes from the steam, leading to a failed sterilization cycle even if the autoclave reaches the correct temperature and pressure.
Critical Steps Before Starting a Cycle
Proper preparation of both the autoclave and its contents is the most important factor in a successful cycle. Rushing these steps is the most common cause of failure.
Preparing the Chamber
Before loading, ensure the autoclave's drain screen is clean and the drain valve is closed. Add the correct amount of deionized or distilled water according to the manufacturer's level indicator to ensure sufficient steam can be generated.
Loading Items Correctly
Never overcrowd the chamber. Items should be loaded loosely to create pathways for steam to circulate freely. Place containers like flasks and beakers on their sides, and ensure any lids are ajar or removed to prevent air pockets and allow steam to enter.
Segregating Loads
Always process clean items (like glassware and media) and biohazardous waste in separate cycles. This prevents cross-contamination and ensures waste is treated with the appropriate decontamination cycle, which may have different time and temperature parameters.
Common Pitfalls and Prohibited Materials
Ignoring safety guidelines and material limitations can lead to failed sterilization, damage to the autoclave, or serious personal injury.
The Danger of Sealed Containers
Never autoclave liquids in a sealed container. As the liquid heats up, it will expand and boil, creating immense pressure inside the container with no means of escape. This can cause the container to explode, damaging the autoclave and posing a significant safety hazard.
Materials That Block Steam or Melt
Avoid materials that are incompatible with steam sterilization. This includes:
- Waterproof substances like oils, waxes, and greases, as steam cannot penetrate them.
- Aluminum foil, which acts as a barrier and prevents steam from reaching the wrapped object's surface.
- Polyethylene (PE) plastic trays, as they have a low melting point and can melt onto the chamber floor, causing costly damage.
The Inefficiency of Overcrowding
Packing the chamber too tightly is the primary cause of failed cycles. Dense loads prevent steam from circulating and penetrating to the center of the load, leaving items in the middle unsterilized. It is always better to run two small, loose loads than one large, dense one.
Making the Right Choice for Your Goal
Your approach to loading should adapt to the material you are processing. The goal is always to maximize steam contact.
- If your primary focus is sterilizing laboratory glassware: Place flasks and beakers on their sides to allow air to escape and steam to enter easily.
- If your primary focus is decontaminating biohazardous waste: Use certified autoclave bags, leave them partially open to permit steam entry, and place them in a secondary, autoclavable tray to contain any potential spills.
- If your primary focus is sterilizing surgical or medical instruments: Disassemble any multi-part instruments and ensure they are arranged in a single layer in a specialized tray or pouch that allows for maximum steam exposure.
Ultimately, successful autoclaving is a function of methodical preparation and a clear understanding of its principles.
Summary Table:
| Key Consideration | Primary Goal |
|---|---|
| Ensure Steam Penetration | Achieve true sterilization by allowing steam to contact all surfaces. |
| Prepare Chamber & Load Correctly | Prevent cycle failure and ensure even heat distribution. |
| Avoid Prohibited Materials (e.g., sealed containers, waterproof substances) | Prevent equipment damage and safety hazards. |
| Segregate Loads (clean vs. biohazardous) | Prevent cross-contamination and use correct cycle parameters. |
Need reliable autoclaves or expert advice for your laboratory?
KINTEK specializes in high-performance lab equipment and consumables, providing the precise tools you need for effective sterilization. Our autoclaves are designed for safety, efficiency, and consistent results, helping you maintain the highest standards in your work.
Contact our experts today to find the perfect autoclave solution for your specific laboratory needs!
Visual Guide
Related Products
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Desktop Fast High Pressure Laboratory Autoclave Sterilizer 16L 24L for Lab Use
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Test Sieves and Sieving Machines
People Also Ask
- What should be autoclaved in a lab? A Guide to Safe and Effective Sterilization
- How often should an autoclave be serviced? A Risk-Based Guide to Sterilization Compliance
- What can you use instead of autoclave? Find the Right Sterilization Method for Your Materials
- What is a "standard load" in the context of autoclave load validation? Define Your Sterilization Ceiling
- What critical functions do zirconia sleeves and gaskets perform in autoclave cracking tests? Ensure Data Precision
- Why is a PTFE-lined autoclave necessary for Na-Ce-modified-SBA-15 catalyst aging? Ensuring Structural Integrity
- What is the lowest temperature for sterilization? Methods for Heat-Sensitive Materials
- What is the temperature that must be reached in an autoclave? Achieve Guaranteed Sterility with the Right Parameters



















