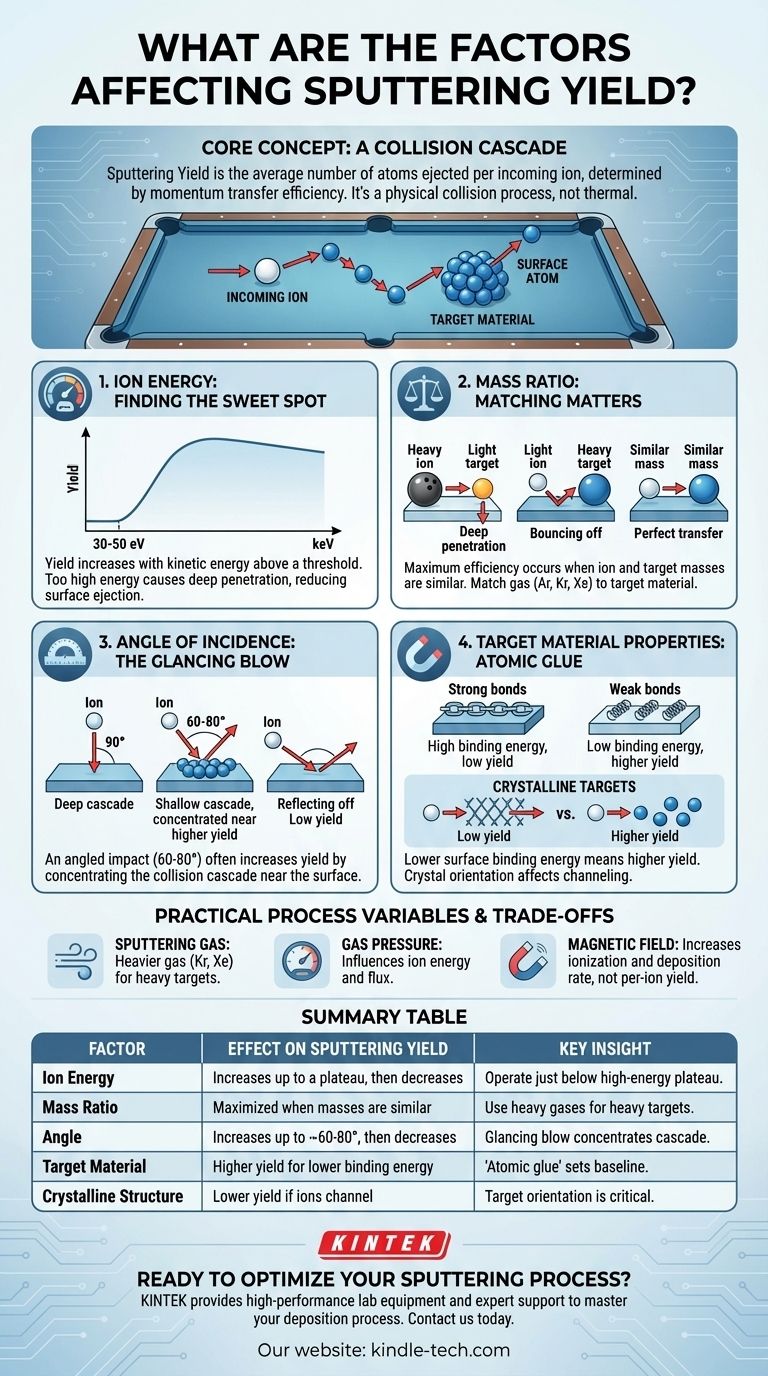
At its core, sputtering yield is determined by the efficiency of momentum transfer from an incoming ion to the atoms of a target material. The primary factors controlling this are the energy and mass of the bombarding ion, the angle of impact, and the properties of the target material itself, specifically the energy binding its surface atoms together.
Sputtering is a physical collision process, not a thermal one. The goal is to maximize the energy transferred to the target's surface atoms in a way that ejects them. Understanding how each process variable influences this energy transfer is the key to controlling your deposition rate and film quality.

The Core Physics: A Collision Cascade
Sputtering is best understood as a microscopic game of billiards. An incoming ion (the "cue ball") strikes atoms within the target material, creating a chain reaction or "collision cascade."
When this cascade of moving atoms reaches the surface with enough energy, surface atoms can be knocked loose and ejected. The sputtering yield is simply the average number of atoms ejected per incoming ion.
Analyzing the Key Factors
To control the sputtering yield, you must manipulate the variables that govern the efficiency of this collision cascade.
Ion Energy: Finding the Sweet Spot
The kinetic energy of the bombarding ions is a critical control parameter. There is a minimum energy threshold, typically 30-50 eV, required to overcome the forces holding the target atoms in place.
Below this threshold, no sputtering occurs. Above it, the yield generally increases with energy.
However, at very high energies (e.g., above a few keV), the yield begins to plateau or even decrease. This is because extremely high-energy ions penetrate too deeply into the target, depositing their energy far below the surface where it cannot contribute to ejecting atoms.
Mass Ratio: The Importance of Matching
The efficiency of momentum transfer depends heavily on the relative masses of the ion and the target atom.
Maximum energy transfer occurs when the masses are approximately equal. Think of one billiard ball striking another—the energy transfer is nearly perfect.
If a heavy ion (like a bowling ball) hits a light target atom (a ping-pong ball), the light atom is ejected with high velocity, but the ion continues deep into the target, wasting energy. Conversely, a light ion hitting a heavy target atom will simply bounce off, transferring very little momentum.
Angle of Incidence: The Glancing Blow
A perpendicular impact (90°) is not always the most efficient angle for sputtering.
Often, an angled impact (typically 60-80° from normal) increases the sputter yield. This is because the collision cascade is concentrated closer to the surface, making it more likely for an atom to be ejected.
However, at very shallow angles, the ion is more likely to simply reflect off the surface without initiating a significant cascade, causing the yield to drop off sharply.
Target Material Properties: The Atomic Glue
The inherent properties of the target material set the baseline for the sputtering process.
The most important factor is the surface binding energy. This is the amount of energy required to remove an atom from the surface. Materials with lower surface binding energies will have a higher sputtering yield, as less energy is needed to eject an atom.
For crystalline targets, the orientation of the crystal lattice relative to the ion beam is also crucial. If ions enter along an open crystal channel ("channeling"), they travel deeper into the material with fewer collisions, significantly reducing the sputter yield.
Understanding the Trade-offs and Process Variables
The core physical principles are controlled through practical machine settings. Understanding the connection is vital.
Choosing the Right Sputtering Gas
The choice of gas (e.g., Argon, Krypton, Xenon) directly sets the ion mass. Argon is a common, cost-effective choice. However, to maximize the yield for heavy targets like gold or platinum, a heavier and more expensive gas like Krypton or Xenon is more effective due to better mass matching.
Gas Pressure
Gas pressure influences both ion energy and flux. Lower pressure increases the "mean free path" of ions, allowing them to accelerate to higher energies before striking the target. However, too low a pressure can lead to an unstable plasma.
Magnetic Field Strength
In magnetron sputtering, a magnetic field is used to trap electrons near the target surface. This dramatically increases the ionization efficiency of the sputtering gas, creating a denser plasma and a higher flux of ions hitting the target. This increases the overall deposition rate but does not change the yield per individual ion.
Making the Right Choice for Your Goal
Your optimal parameters depend entirely on what you are trying to achieve.
- If your primary focus is maximizing deposition rate: Use a heavy sputter gas (Krypton/Xenon) for heavy targets, operate at an energy just below the "plateau" point, and optimize the ion's angle of incidence.
- If your primary focus is sputtering a light or delicate material: Choose a lighter sputter gas (Neon/Argon) for better mass-matching and use just enough energy to exceed the sputtering threshold to minimize subsurface damage.
- If your primary focus is process repeatability: Meticulously control your gas pressure, power (which dictates ion energy), and target temperature, as these factors directly govern the stability of your yield.
Mastering these factors transforms sputtering from a black box into a precisely controllable engineering process.
Summary Table:
| Factor | Effect on Sputtering Yield | Key Insight |
|---|---|---|
| Ion Energy | Increases up to a plateau, then decreases | Operate just below the high-energy plateau for maximum efficiency. |
| Mass Ratio (Ion/Target) | Maximized when masses are similar | Use heavy gases (Kr, Xe) for heavy targets; light gases (Ne, Ar) for light targets. |
| Angle of Incidence | Increases up to ~60-80°, then decreases sharply | A glancing blow concentrates the collision cascade near the surface. |
| Target Material (Surface Binding Energy) | Higher yield for materials with lower binding energy | The "atomic glue" strength sets the baseline for the process. |
| Crystalline Structure | Yield is lower if ions channel into the crystal lattice | Target orientation relative to the beam is critical for crystalline materials. |
Ready to optimize your sputtering process for maximum yield and superior film quality?
The factors detailed above are the levers you control to achieve precise, repeatable results. At KINTEK, we specialize in providing the high-performance lab equipment and expert support you need to master your deposition process.
Whether you are focused on maximizing deposition rate, working with delicate materials, or ensuring process repeatability, our range of sputtering systems and consumables is designed to meet your specific laboratory requirements.
Contact us today to discuss how our solutions can enhance your research and development. Let's turn your sputtering process from a challenge into a competitive advantage.
Get in touch with our experts now →
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Evaporation Boat for Organic Matter
- Electron Beam Evaporation Coating Oxygen-Free Copper Crucible and Evaporation Boat
- Hemispherical Bottom Tungsten Molybdenum Evaporation Boat
People Also Ask
- Why does PECVD commonly use RF power input? For Precise Low-Temperature Thin Film Deposition
- What is the plasma CVD process? Achieve Low-Temperature Thin Film Deposition
- How are PECVD and CVD different? A Guide to Choosing the Right Thin-Film Deposition Process
- How does RF power create plasma? Achieve Stable, High-Density Plasma for Your Applications
- What is an example of PECVD? RF-PECVD for High-Quality Thin Film Deposition



















