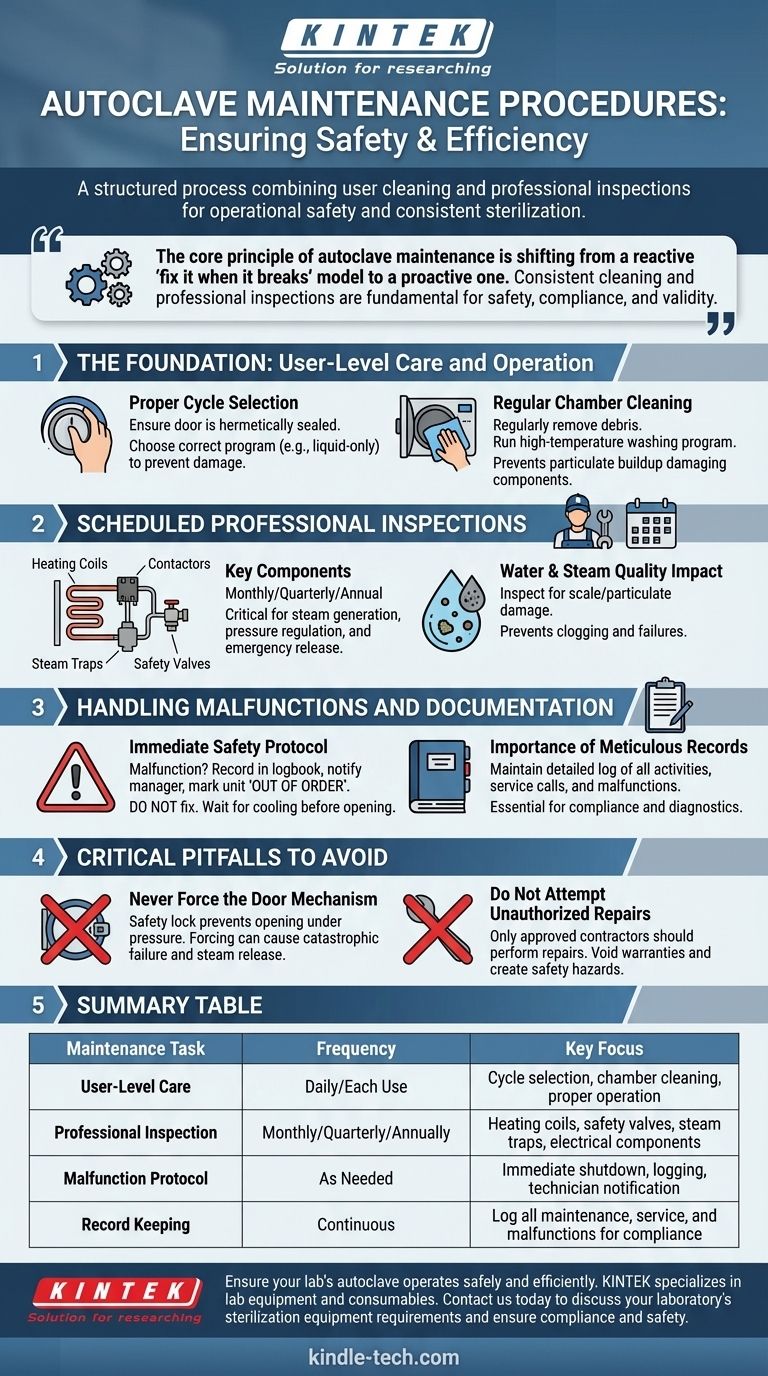
Autoclave maintenance is a structured process combining routine user cleaning with scheduled professional inspections of key components like heating coils, safety valves, and steam traps. The goal is to ensure both operational safety and the consistent effectiveness of the sterilization cycle. Adherence to a documented maintenance schedule is critical for preventing equipment damage and ensuring user safety.
The core principle of autoclave maintenance is shifting from a reactive "fix it when it breaks" model to a proactive one. Consistent cleaning and professional inspections are not just about longevity; they are fundamental requirements for ensuring safety, compliance, and the validity of every sterilization cycle.

The Foundation: User-Level Care and Operation
Proper daily use and cleaning form the first line of defense in maintaining an autoclave's health and performance. These are tasks that should be integrated into the standard operating procedure for any user.
Proper Cycle Selection
Before starting any cycle, ensure the door is fully closed and the locking mechanism is hermetically sealed without using excessive force.
Choosing the correct program is a crucial, often overlooked, aspect of maintenance. Using a liquid-only program for liquids, for example, prevents boil-overs that can block drains and damage sensors.
Regular Chamber Cleaning
The vacuum chamber must be cleaned regularly to remove debris and residue. A dedicated high-temperature washing program should be run periodically, if available.
This simple step prevents the buildup of particulates that can be carried by steam, potentially damaging sensitive components like valves and steam traps.
Scheduled Professional Inspections
While user cleaning is essential, the core mechanical and electrical components require periodic inspection by qualified technicians. These tasks are typically performed on a monthly, quarterly, or annual basis.
Key Components for Inspection
A professional maintenance schedule should include thorough checks of the heating coils, contactors, steam traps, and safety valves.
These components control the generation of steam, regulation of pressure, and emergency safety releases. Their failure can lead to ineffective sterilization or a critical safety incident.
The Impact of Water and Steam Quality
Inspections are also vital for identifying early signs of damage from poor water quality or particulates in the steam supply.
Scale buildup and particulate matter can clog valves, insulate heating elements, and cause catastrophic failures over time.
Handling Malfunctions and Documentation
A clear protocol for handling malfunctions is just as important as preventative maintenance. This ensures user safety and provides critical information for service technicians.
Immediate Safety Protocol
If an autoclave malfunctions, immediately record the issue in the logbook, notify the laboratory manager, and clearly mark the unit as out of order.
Do not attempt to diagnose or fix the problem yourself. Even if a cycle is aborted, only remove the contents after the machine has completely cooled down.
The Importance of Meticulous Records
Maintain a detailed on-site log of all maintenance activities, service calls, and malfunctions. This creates a complete history of the equipment's performance and service.
This documentation is essential for regulatory compliance and helps technicians diagnose recurring problems more efficiently.
Critical Pitfalls to Avoid
Simple operator errors can lead to dangerous situations or costly repairs. Avoiding these common mistakes is a key part of any autoclave maintenance and safety program.
Never Force the Door Mechanism
The safety lock mechanism is designed to prevent the door from being opened under pressure. Never attempt to force it open.
Forcing the door can cause catastrophic failure of the lock and result in the explosive release of superheated steam.
Do Not Attempt Unauthorized Repairs
Only contractors approved by the manufacturer should perform maintenance and repairs. Attempting to fix the unit yourself can void warranties and create significant safety hazards.
Keep the contact information for your approved maintenance contractor posted conveniently near the autoclave.
Making the Right Choice for Your Goal
A robust maintenance plan ensures your autoclave is not just functional, but safe, reliable, and compliant. Your focus will determine which aspects of maintenance you prioritize.
- If your primary focus is daily operational safety: Prioritize user training on proper cycle selection and the strict "do not force the door" and "report all malfunctions" protocols.
- If your primary focus is long-term equipment reliability: Emphasize strict adherence to the scheduled professional inspection of coils, valves, and traps to prevent failures before they happen.
- If your primary focus is compliance and validation: Center your efforts on meticulous, consistent record-keeping for every cleaning cycle, maintenance visit, and malfunction report.
Ultimately, a consistent and well-documented maintenance routine is the foundation upon which every successful sterilization process is built.
Summary Table:
| Maintenance Task | Frequency | Key Focus |
|---|---|---|
| User-Level Care | Daily / Each Use | Cycle selection, chamber cleaning, proper operation |
| Professional Inspection | Monthly / Quarterly / Annually | Heating coils, safety valves, steam traps, electrical components |
| Malfunction Protocol | As Needed | Immediate shutdown, logging, technician notification |
| Record Keeping | Continuous | Log all maintenance, service, and malfunctions for compliance |
Ensure your lab's autoclave operates safely and efficiently. Proper maintenance is critical for sterilization validity and user protection. KINTEK specializes in lab equipment and consumables, serving laboratory needs. Our experts can help you establish a robust maintenance schedule or provide reliable service for your autoclaves. Contact us today to discuss your laboratory's sterilization equipment requirements and ensure compliance and safety.
Visual Guide
Related Products
- Portable High Pressure Laboratory Autoclave Steam Sterilizer for Lab Use
- Laboratory Sterilizer Lab Autoclave Herbal Powder Sterilization Machine for Plant
- Laboratory Sterilizer Lab Autoclave Pulsating Vacuum Desktop Steam Sterilizer
- Laboratory High Pressure Steam Sterilizer Vertical Autoclave for Lab Department
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
People Also Ask
- What are the applications of autoclave? Essential Sterilization for Medical, Lab, and Industrial Needs
- What are the safety concerns of autoclave? Managing Heat, Pressure, and Steam Risks
- What is the function of a laboratory autoclave in dilute acid pretreatment? Unlock Biomass Energy Efficiently
- What is the primary function of a laboratory autoclave in the pre-treatment of medical plastic waste for liquid fuel?
- How do you use an autoclave in a microbiology lab? Master Sterilization for Lab Safety & Accuracy
- What materials cannot be autoclaved and why? Protect Your Lab Equipment and Ensure Sterility
- What critical functions do zirconia sleeves and gaskets perform in autoclave cracking tests? Ensure Data Precision
- For what purpose are high-pressure autoclaves used in the synthesis of additives for composite polymer electrolytes?



















