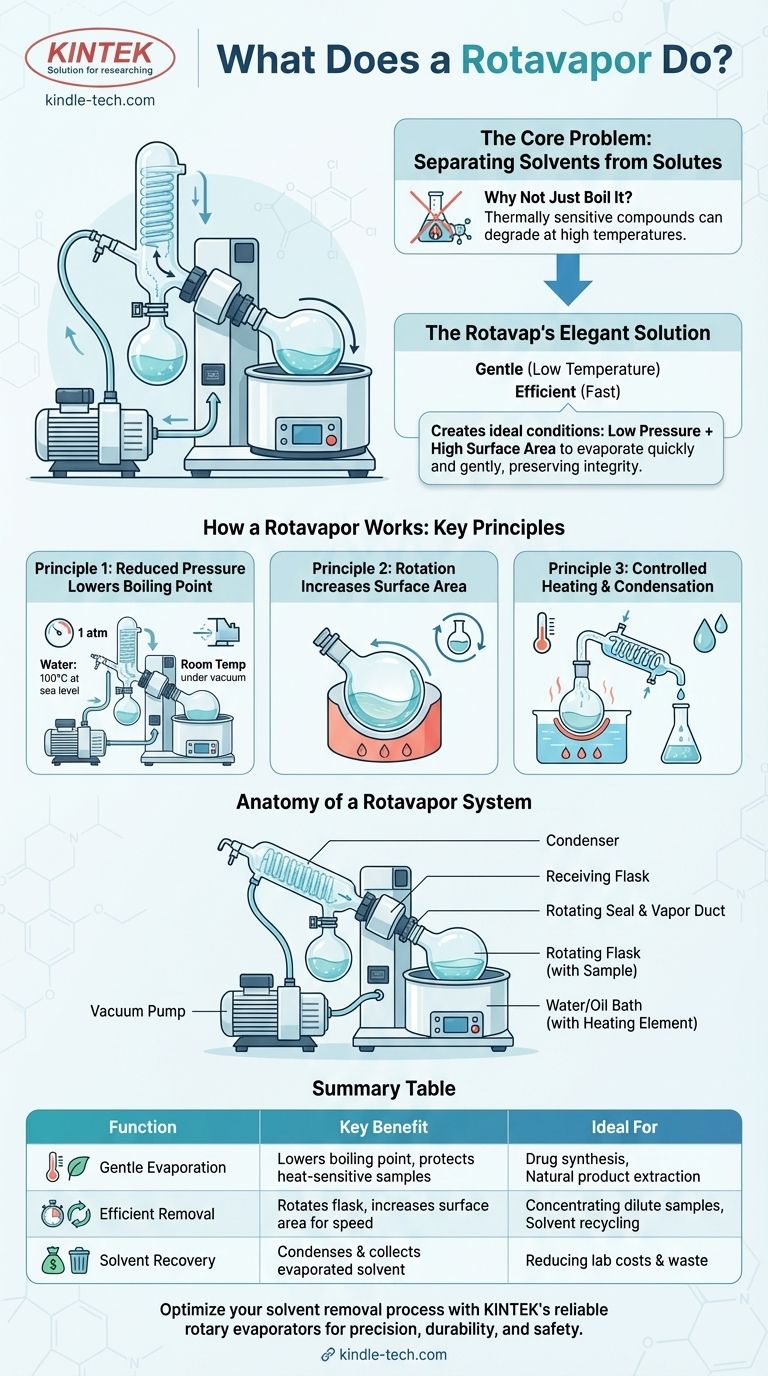
At its core, a rotary evaporator, or "rotavap," is a laboratory instrument used for the efficient and gentle removal of solvents from samples by evaporation. It achieves this by reducing the pressure in the system, which lowers the solvent's boiling point, and by rotating the sample flask to increase the surface area for faster evaporation. This combination allows for rapid solvent removal without exposing the sample to damaging high temperatures.
A rotavap doesn't just boil off a solvent; it creates the ideal physical conditions—low pressure and high surface area—to evaporate it quickly and gently, preserving the integrity of the desired compound left behind.

The Core Problem: Separating Solvents from Solutes
In nearly every chemical process, from synthesizing a new drug to extracting a natural flavor, the compound you want is often left dissolved in a liquid solvent you don't. The fundamental challenge is to remove that solvent without harming your valuable product.
Why Not Just Boil It?
Simple heating, or distillation, is the most basic way to evaporate a liquid. However, many valuable chemical and biological compounds are thermally sensitive.
Exposing them to the high temperatures required to boil a solvent at atmospheric pressure can cause them to decompose or degrade, destroying the very thing you're trying to isolate.
The Rotavap's Elegant Solution
The rotavap solves this problem by manipulating two key physical principles simultaneously. It makes evaporation both gentle (low temperature) and efficient (fast).
How a Rotavapor Works: The Key Principles
The genius of the rotavap lies in how it combines several simple physical laws into one highly effective system.
Principle 1: Reduced Pressure Lowers the Boiling Point
The boiling point of a liquid is the temperature at which its vapor pressure equals the pressure of the surrounding environment. By attaching a vacuum pump, a rotavap drastically reduces the pressure inside the glassware.
This lower pressure means the solvent can boil at a much lower, gentler temperature. For example, water boils at 100°C (212°F) at sea level, but under a strong vacuum, it can boil at room temperature.
Principle 2: Rotation Increases Surface Area
A stationary pool of liquid only evaporates from its top surface. The rotavap's motor constantly spins the sample flask, which is angled into a heated bath.
This rotation spreads the sample into a thin, continuously refreshed film on the inner wall of the flask. This dramatically increases the surface area available for evaporation, making the process much faster and more uniform.
Principle 3: Controlled Heating and Condensation
The rotating flask is lowered into a water or oil bath that provides gentle, consistent heat. This energy fuels the evaporation that the low pressure and high surface area enable.
As the solvent evaporates, the vapor travels into a condenser—a glass coil chilled with circulating cold water or coolant. The cold surface causes the solvent vapor to turn back into a liquid, where it drips down and is collected in a separate receiving flask. This prevents solvent vapors from damaging the vacuum pump and allows for solvent recovery.
Anatomy of a Rotavapor System
A complete rotavap setup consists of several key components working in concert.
The Rotating Flask
This flask holds the initial solution of your compound and the solvent you wish to remove.
The Water Bath
This basin of heated water provides the gentle, uniform thermal energy needed for evaporation without creating dangerous "hot spots."
The Vapor Duct and Motor
The motor spins the flask. A specialized vapor duct and rotating seal allow the flask to spin while remaining connected to the rest of the system under a continuous vacuum.
The Condenser
This is a set of glass coils, typically arranged vertically or diagonally, through which a coolant is circulated. It provides the cold surface needed to re-liquefy the solvent vapor.
The Receiving Flask
Positioned below the condenser, this flask collects the pure, condensed solvent as it drips down, separating it from your original sample.
The Vacuum Pump
The heart of the operation, the vacuum pump removes air from the system to reduce the internal pressure, enabling low-temperature boiling.
Understanding the Trade-offs and Considerations
While powerful, a rotavap is not a magic bullet and requires proper technique to be used effectively and safely.
Solvent Bumping
If the vacuum is applied too quickly or the heat is too high, the solvent can boil violently in an event called bumping. This can splash your sample out of the rotating flask and into the condenser, leading to loss of product. The rotation of the flask is a key defense against bumping.
Foaming Samples
Some samples, particularly those containing proteins or surfactants, may foam excessively under vacuum. This foam can easily travel into the condenser, causing contamination and sample loss.
System Leaks
The entire process depends on maintaining a good vacuum. Any leaks in the glassware joints or rotating seal will raise the pressure, increasing the required boiling temperature and drastically reducing the system's efficiency.
Incompatible Solvents
Very high-boiling point solvents, like DMSO or water, can be slow and difficult to remove even with a good rotavap. For removing water from sensitive samples, a lyophilizer (freeze-dryer) is often a superior tool.
Key Applications in the Lab
A rotavap is a workhorse instrument found in virtually every organic chemistry lab for several primary goals.
- If your primary focus is purifying a reaction product: Use the rotavap to remove the reaction solvent before performing further purification like column chromatography.
- If your primary focus is concentrating a dilute sample: Gently evaporate the excess solvent to increase the concentration of your compound without degrading it.
- If your primary focus is recycling expensive solvents: The rotavap efficiently captures and collects the evaporated solvent in the receiving flask, allowing it to be reused.
- If your primary focus is isolating a natural product from an extract: Use it to carefully remove the extraction solvent (e.g., ethanol, hexane) to obtain the crude natural product for further study.
Ultimately, the rotavap is an indispensable tool that grants chemists precise control over solvent removal, accelerating research and discovery.
Summary Table:
| Function | Key Benefit | Ideal For |
|---|---|---|
| Gentle Evaporation | Lowers boiling point under vacuum to protect heat-sensitive samples | Drug synthesis, natural product extraction |
| Efficient Removal | Rotates flask to increase surface area for faster evaporation | Concentrating dilute samples, solvent recycling |
| Solvent Recovery | Condenses and collects evaporated solvent for reuse | Reducing costs and waste in the lab |
Optimize your solvent removal process with KINTEK's reliable rotary evaporators. Our lab equipment is designed for precision, durability, and safety, helping you protect valuable compounds and streamline your workflow. Contact us today to find the perfect rotavap solution for your laboratory needs!
Visual Guide
Related Products
- Circulating Water Vacuum Pump for Laboratory and Industrial Use
- Oil Free Diaphragm Vacuum Pump for Laboratory and Industrial Use
- 30T 40T Split Automatic Heated Hydraulic Press Machine with Heated Plates for Laboratory Hot Press
- Electric Heated Hydraulic Vacuum Heat Press for Lab
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
People Also Ask
- What is an ultra-low temperature freezer? Protect Your Most Valuable Biological Samples
- What is ethylene cracking furnace? The High-Temperature Heart of Petrochemical Production
- What is the sputtering voltage of a magnetron? Optimize Your Thin Film Deposition Process
- Are biomass fuels sustainable? Uncover the truth behind carbon neutrality and lifecycle impacts.
- What is the difference between fast pyrolysis and slow pyrolysis of biochar? Optimize Your Biomass Conversion Strategy
- What is the science behind sintering? Mastering the Thermal Process for Stronger Materials
- What is the efficiency of biomass to electricity conversion? Unlock 20-40% Electrical & 80%+ Overall Efficiency
- What is the product yield of pyrolysis? Control Your Output for Biochar, Bio-oil, or Syngas



















