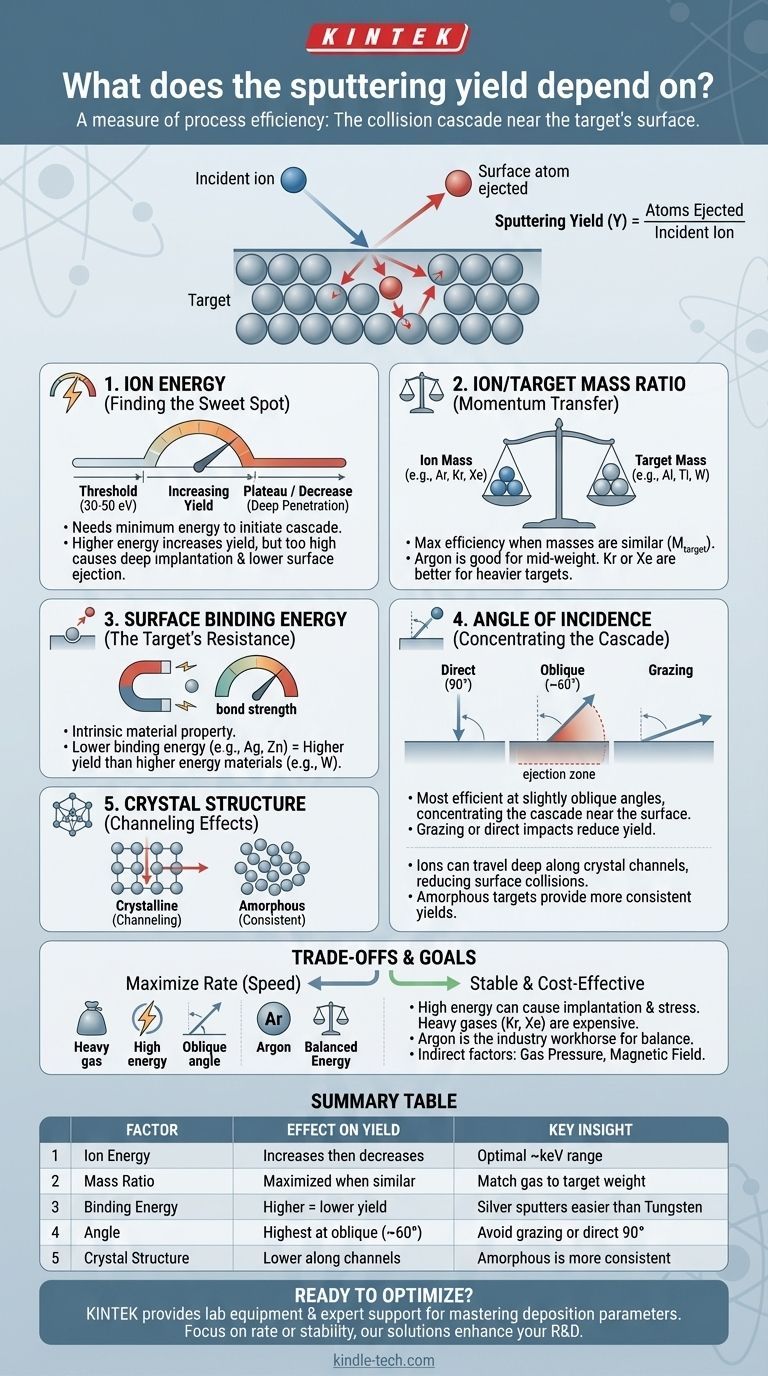
At its core, sputtering yield is a measure of process efficiency. It is the average number of atoms ejected from a target material for each single ion that strikes its surface. This yield is not a fixed value; it is dictated by the fundamental physics of the collision, primarily depending on the incoming ion's energy and mass, the target atom's mass and surface binding energy, and the angle of impact.
Sputtering is fundamentally a game of momentum transfer. The key to understanding sputtering yield is to see it not as a list of independent variables, but as the result of a single event: a collision cascade near the target's surface. Your ability to control the yield depends entirely on how you manipulate the factors that govern the efficiency of that energy transfer.
The Physics of the Collision Cascade
Sputtering happens when an incident ion strikes a target with enough energy to initiate a chain reaction of atomic collisions. This "collision cascade" works its way back to the surface, and if an atom at the surface gains enough energy to overcome its bonds, it is ejected. The sputter yield quantifies the success of this process.
The Role of Ion Energy: Finding the Sweet Spot
To eject a target atom, an incoming ion must first overcome the material's surface binding energy. This requires a minimum kinetic energy, typically between 30 and 50 electron volts (eV).
Below this threshold, the ions lack the force to initiate a productive cascade, and sputtering does not occur.
Above the threshold, the sputter yield increases significantly with ion energy. More energy means a more violent and widespread collision cascade, increasing the probability of ejecting surface atoms.
However, this trend does not continue indefinitely. At very high energies (often above a few thousand eV), the incoming ions penetrate too deeply into the target. The collision cascade's energy is deposited far below the surface, making it less likely for a surface atom to be ejected. This causes the sputter yield to plateau or even decrease.
Momentum Transfer: Matching Ion and Target Mass
The efficiency of any collision depends on the masses of the colliding objects. The same is true at the atomic scale. The ratio of the ion's mass to the target atom's mass is a critical factor in determining how much momentum is transferred.
Maximum energy transfer occurs when the masses are approximately equal. This is why Argon (atomic mass ~40 amu) is a common and effective sputtering gas for many mid-weight metals like Aluminum (~27 amu) or Titanium (~48 amu).
For heavier target atoms, using a heavier sputtering gas like Krypton (~84 amu) or Xenon (~131 amu) will result in more efficient momentum transfer and a significantly higher sputter yield.
The Target's Resistance: Surface Binding Energy
The surface binding energy is the energy that holds atoms to the surface of the target. It is an intrinsic property of the target material itself.
Materials with a lower surface binding energy are "easier" to sputter. Their atoms require less energy to be ejected from the surface, which translates directly to a higher sputtering yield under the same conditions. For example, metals like zinc and silver have lower binding energies and higher sputter yields than tungsten.
Geometric and Structural Influences
Beyond the core physics of the collision, the geometry of the interaction also plays a significant role.
The Angle of Incidence
Sputtering is generally most efficient at a slightly oblique angle of incidence, not a direct 90-degree impact.
When an ion strikes the surface at an angle, the collision cascade is concentrated closer to the surface. This increases the likelihood that dislodged atoms will be ejected rather than simply displaced deeper into the target.
However, at very shallow (grazing) angles, the ion is more likely to simply scatter off the surface, which reduces the sputter yield again.
Crystalline vs. Amorphous Targets
For targets with a crystalline structure, the orientation of the crystal axes relative to the ion beam matters.
If the ions strike along an open "channel" in the crystal lattice, they can travel deep into the material with very few collisions. This phenomenon, known as channeling, significantly reduces the number of surface collisions and therefore lowers the sputter yield.
Understanding the Trade-offs
Optimizing for the highest possible yield is not always the best strategy. The choices you make involve practical and financial trade-offs.
High Energy Isn't Always Better
Pushing ion energy to the maximum for higher yield can have negative consequences. Extremely high-energy ions can become embedded in the target or the growing film (ion implantation), which can introduce impurities and stress. It also requires more power and can lead to excessive target heating.
The Gas Mass Dilemma
While heavier noble gases like Krypton and Xenon provide a much higher sputter yield, they are also significantly more expensive than Argon. For most industrial applications, Argon provides the best balance of performance and cost-effectiveness, making it the industry workhorse.
Indirect Process Parameters
Factors like gas pressure and magnetic field strength (in magnetron sputtering) do not set the sputter yield directly. Instead, they are the control knobs used to influence the primary factors. Increasing gas pressure, for example, can reduce the average ion energy due to more gas-phase collisions, which can lower the yield.
Making the Right Choice for Your Goal
Your approach to controlling sputter yield should be dictated by your end goal, whether it's speed, cost, or film quality.
- If your primary focus is maximizing deposition rate: Use a heavy sputtering gas (if cost permits), operate at the optimal energy just before the yield curve plateaus, and use a slightly off-normal angle of incidence.
- If your primary focus is process stability and cost-effectiveness: Use Argon gas, as it provides a robust and economical solution for a wide range of common target materials.
- If you are sputtering a single-crystal target: Be aware of the target's orientation relative to the ion source, as channeling effects can cause unexpected drops in your sputtering rate.
Ultimately, mastering sputter yield is about controlling the transfer of energy at the atomic scale to achieve your specific material goals.
Summary Table:
| Factor | Effect on Sputtering Yield | Key Insight |
|---|---|---|
| Ion Energy | Increases to a plateau, then decreases | Optimal energy is typically in the keV range. |
| Ion/Target Mass Ratio | Maximized when masses are similar | Argon is ideal for mid-weight metals; use Kr or Xe for heavier targets. |
| Surface Binding Energy | Higher energy = lower yield | Materials like silver sputter more easily than tungsten. |
| Angle of Incidence | Highest at oblique angles (~60°) | Grazing or direct (90°) impacts reduce efficiency. |
| Crystal Structure | Lower yield along crystal channels | Amorphous materials provide more consistent yields. |
Ready to Optimize Your Sputtering Process?
Understanding sputtering yield is the first step to achieving precise, high-quality thin films. KINTEK specializes in providing the lab equipment and expert support you need to master your deposition parameters.
Whether you're focused on maximizing deposition rate with heavy gases or ensuring cost-effective, stable processes with argon, our range of sputtering systems and consumables is designed to meet your specific laboratory requirements.
Let's discuss your application. Contact our experts today to explore how our solutions can enhance your research and development outcomes.
Visual Guide
Related Products
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
People Also Ask
- What is the specific function of the metal filament in HF-CVD? Key Roles in Diamond Growth
- What machine is used to make lab-grown diamonds? Discover the HPHT & CVD Technologies
- How are reactants introduced into the reaction chamber during a CVD process? Mastering Precursor Delivery Systems
- What is the role of the HF-CVD system in preparing BDD electrodes? Scalable Solutions for Boron-Doped Diamond Production
- What is the hot filament chemical vapour deposition of diamond? A Guide to Synthetic Diamond Coating



















