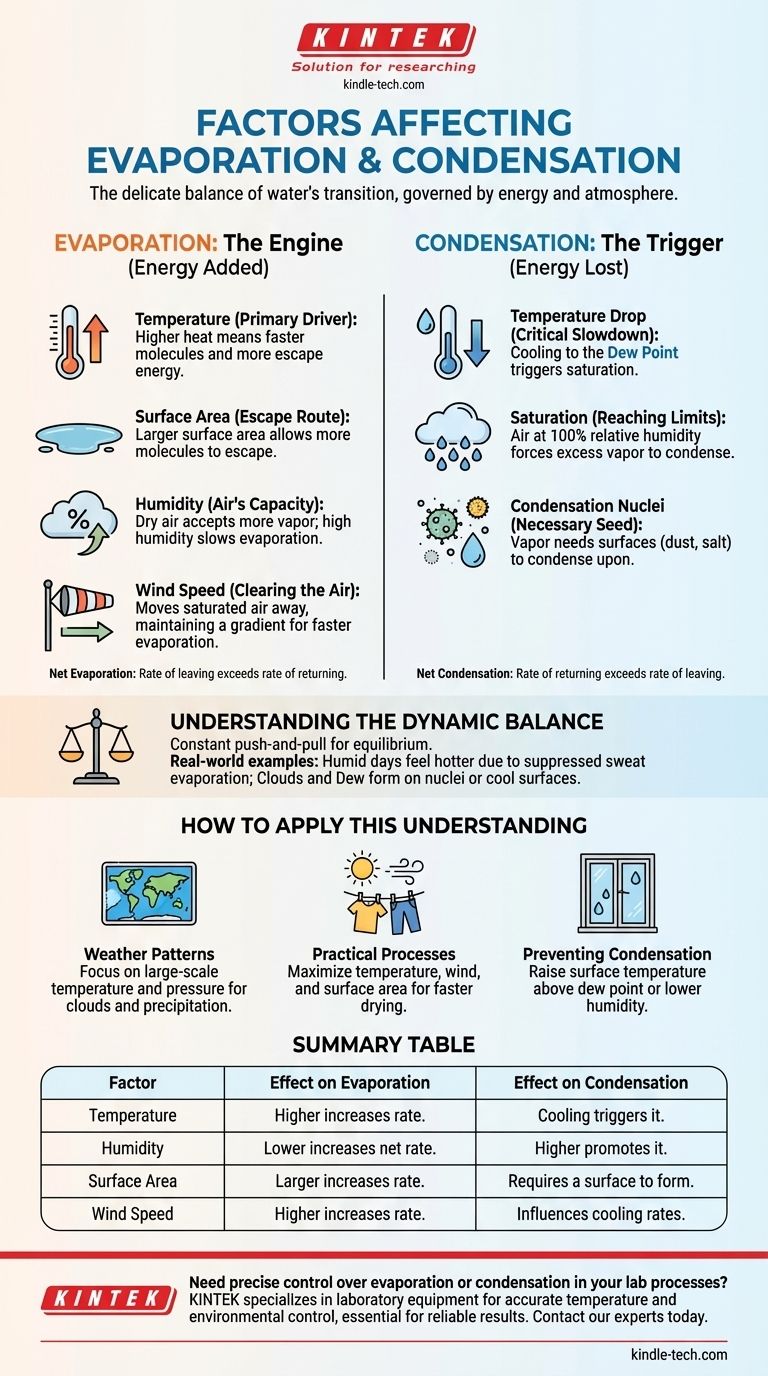
The transition of water between liquid and gas is governed by a delicate balance of energy and atmospheric conditions. For evaporation, the key factors are temperature, surface area, humidity, and wind speed. For condensation, the critical factors are a drop in temperature to the dew point, the amount of water vapor already in the air, and the presence of surfaces known as condensation nuclei.
Evaporation is fundamentally an energy-driven process that allows water to escape, while condensation is the release of that energy as water returns to a liquid state. Understanding this exchange of energy is the key to understanding why and when each process occurs.

The Engine of Evaporation: Adding Energy and Space
Evaporation is the process where liquid water absorbs enough energy to transition into a gaseous state, or water vapor. The rate at which this happens is controlled by several interconnected factors.
Temperature: The Primary Driver
Heat is a form of energy. When the temperature of water increases, its molecules move faster and with more energy.
This increased kinetic energy allows more molecules to overcome the bonds holding them together in the liquid state and escape from the surface into the air as vapor.
Surface Area: The Escape Route
Evaporation only happens at the surface of a liquid. The larger the surface area exposed to the air, the more molecules are in a position to escape.
This is why a puddle of water spread thin on the pavement evaporates much faster than the same amount of water in a deep glass.
Humidity: The Air's Capacity for Water
Relative humidity measures how much water vapor is currently in the air compared to the maximum amount it could hold at that temperature.
If the air is already saturated with water (100% relative humidity), there is little "room" for more vapor. This significantly slows down the rate of net evaporation. Dry air, by contrast, readily accepts more water vapor.
Wind Speed: Clearing the Air
As water evaporates, it creates a thin layer of humid air directly above the water's surface. This can slow down further evaporation.
Wind acts to blow this saturated layer away, replacing it with drier air. This maintains a steep concentration gradient, encouraging a faster and more continuous rate of evaporation.
The Trigger for Condensation: Losing Energy and Finding a Surface
Condensation is the reverse process of evaporation. It occurs when water vapor in the air cools and changes back into liquid water, releasing the energy it absorbed during evaporation.
Temperature Drop: The Critical Slowdown
For condensation to occur, the air must cool to its dew point. This is the temperature at which the air becomes fully saturated with the water vapor it contains.
As air cools, its molecules slow down. Once they slow enough, the weak attractions between them can pull them back together into liquid droplets.
The Role of Saturation
Condensation is a direct result of air reaching 100% relative humidity. At this point, the air simply cannot hold any more water in its gaseous form at its current temperature.
Any further cooling, or the addition of more water vapor, will force some of that vapor to condense into a liquid.
Condensation Nuclei: A Necessary Seed
Water vapor needs a non-gaseous surface to condense upon. In the atmosphere, these are microscopic particles of dust, salt, pollen, or pollutants.
These particles are called condensation nuclei, and they serve as the "seeds" around which cloud droplets form. On the ground, condensation forms as dew on larger surfaces like grass, windows, or a cold can of soda that have cooled below the dew point.
Understanding the Dynamic Balance
Evaporation and condensation are not isolated events. They are in a constant push-and-pull, striving for equilibrium. Recognizing their interplay is crucial to understanding real-world phenomena.
The Concept of Net Evaporation
Even when we see a puddle shrinking, some water vapor from the air is still condensing back into the puddle.
What we observe as "evaporation" is actually net evaporation, meaning the rate of molecules leaving the liquid is greater than the rate of molecules returning. The reverse is true for net condensation.
Why Humid Days Feel Hotter
Our bodies cool down by sweating. The evaporation of that sweat removes heat from our skin.
On a hot, humid day, the high concentration of water vapor in the air suppresses the rate of evaporation. Because our sweat can't evaporate efficiently, we lose our primary cooling mechanism and feel hotter.
How Clouds and Dew Form
These two phenomena perfectly illustrate the principles of condensation. Clouds form when a parcel of air rises, expands, and cools to its dew point high in the atmosphere, condensing on airborne nuclei.
Dew forms when a surface on the ground, like a blade of grass, radiates heat and cools overnight to below the dew point of the surrounding air, causing vapor to condense directly onto it.
How to Apply This Understanding
Your specific goal will determine which factors are most important to consider.
- If your primary focus is on weather patterns: Concentrate on large-scale temperature changes and air pressure systems, which drive air masses to rise and cool, causing condensation (clouds and precipitation).
- If your primary focus is on a practical process (like drying laundry): You should maximize the factors that speed evaporation—increase temperature (sunlight), increase wind (a breeze), and increase surface area (spreading clothes out).
- If your primary focus is on preventing unwanted condensation (on windows or equipment): You must either raise the temperature of the surface so it stays above the dew point or reduce the amount of water vapor in the air (lower the humidity).
By grasping these core principles, you gain the ability to predict and even control the behavior of water in countless natural and engineered systems.
Summary Table:
| Factor | Effect on Evaporation | Effect on Condensation |
|---|---|---|
| Temperature | Higher temperature increases rate. | Cooling to dew point triggers condensation. |
| Humidity | Lower humidity increases net evaporation. | Higher humidity/saturation promotes condensation. |
| Surface Area | Larger area increases rate. | Requires a surface (e.g., nuclei, grass) to form. |
| Wind Speed | Higher speed increases rate by removing humid air. | Less direct impact, but can influence cooling rates. |
Need precise control over evaporation or condensation in your lab processes? KINTEK specializes in laboratory equipment and consumables that provide the accurate temperature and environmental control essential for reliable results. Whether you're developing new materials or running critical analyses, our solutions help you master these fundamental phase changes. Contact our experts today to find the right equipment for your specific laboratory needs.
Visual Guide
Related Products
- Wall Mounted Water Distillation Unit
- Evaporation Boat for Organic Matter
- Evaporation Crucible for Organic Matter
- Laboratory Benchtop Water Circulating Vacuum Pump for Lab Use
- Vacuum Cold Trap Chiller Indirect Cold Trap Chiller
People Also Ask
- Which parameter effect on thin film formation in thermal evaporation? Master the Key Variables for Superior Films
- What is an example of a physical vapor deposition? Discover Sputtering and Thermal Evaporation
- How thermal evaporation is used to deposit a thin metal film? A Simple Guide to High-Purity Coating
- Can you evaporate silver? Master the PVD Process for High-Performance Coatings
- What is the process of thermal evaporation thin film deposition? A Guide to Simple, Cost-Effective PVD
- What is the purpose of vacuum evaporation? Purify Water or Create High-Purity Coatings
- What is the physical deposition technique? A Guide to PVD Coating Methods & Applications
- What metal can evaporate? A Guide to Vapor Pressure and Thin Film Deposition




