In autoclave sterilization, the most critical instruments are not the items being sterilized, but rather the indicators used to verify that the process has succeeded. True sterilization assurance relies on a combination of physical, chemical, and biological indicators to confirm that the necessary conditions for killing microorganisms have been met.
The core principle of autoclave validation is that you cannot assume sterility based on the machine's settings alone. You must use a multi-layered system of physical, chemical, and biological indicators to prove that sterilization conditions were achieved throughout the entire load.

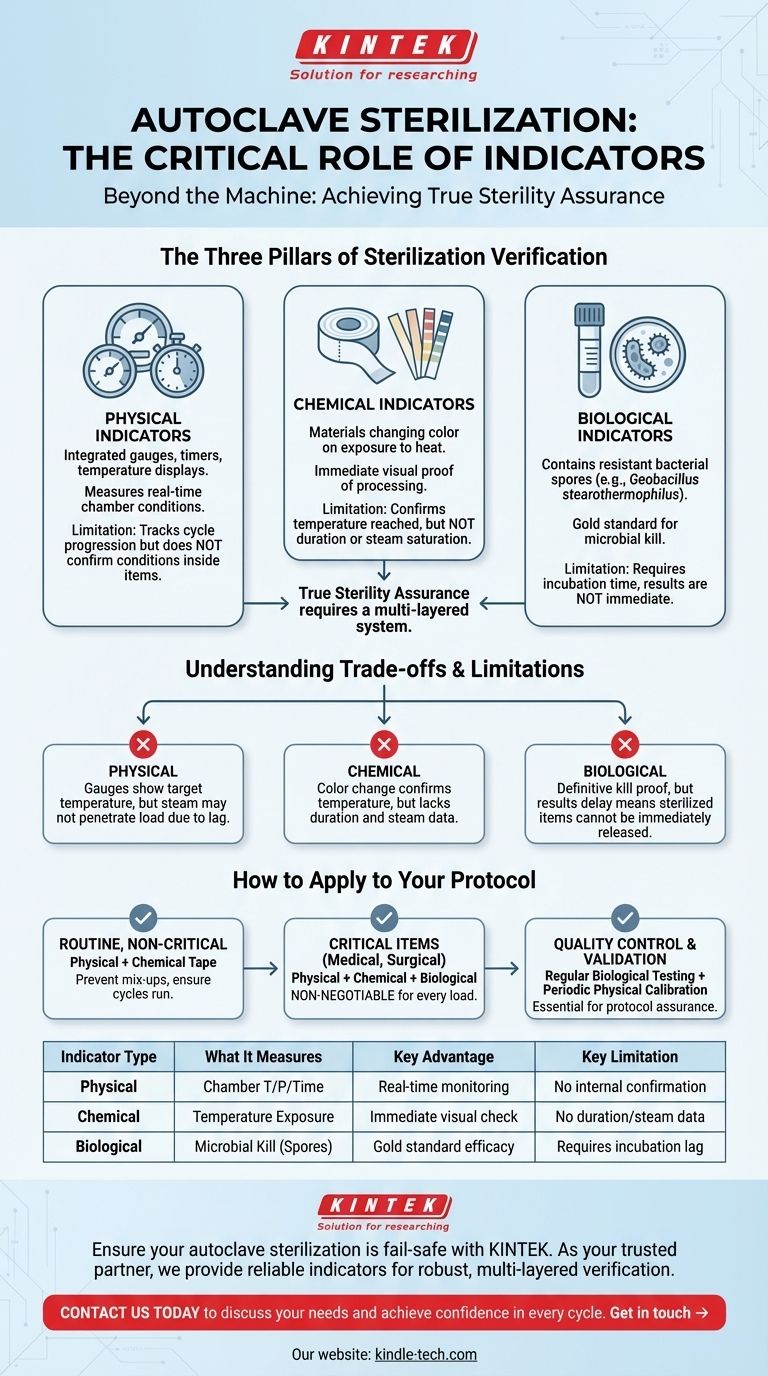
The Three Pillars of Sterilization Verification
To ensure proper autoclave sterilization, a robust monitoring system is essential. This system is built upon three distinct types of indicators, each providing a different piece of the puzzle.
H3: Physical Indicators
Physical indicators are the gauges, timers, and temperature displays integrated into the autoclave itself. They provide a real-time readout of the chamber's conditions.
These instruments track the cycle's progression, confirming that the pre-set time, temperature, and pressure were reached. They are the first line of verification but only report on conditions inside the chamber, not inside the items being sterilized.
H3: Chemical Indicators
Chemical indicators are materials that change color or form when exposed to specific temperatures. The most common example is autoclave indicator tape.
These indicators are placed on the exterior or interior of packs and provide immediate visual proof that an item has been through a heat cycle. They are excellent for distinguishing processed from unprocessed items.
However, they do not confirm the duration or steam saturation necessary for true sterilization.
H3: Biological Indicators
Biological indicators are the gold standard for verifying lethality. They contain highly resistant bacterial spores, typically from Geobacillus stearothermophilus.
If the autoclave cycle is effective, it will kill these resilient spores. After the cycle, the indicator is incubated; if no spores grow, it confirms the sterilization process was successful at a microbial level.
Understanding the Trade-offs and Limitations
Relying on a single type of indicator can create a false sense of security. Each has inherent limitations that must be understood to maintain a sterile environment.
H3: Physical Indicators Can Be Misleading
The autoclave's gauges show that the chamber reached its target temperature, but they cannot confirm that steam penetrated to the core of a densely packed load. A heat transfer lag can mean the items inside never reach the required temperature.
H3: Chemical Indicators Don't Guarantee Sterility
A chemical indicator's color change only confirms that a certain temperature was met. It provides no information about how long that temperature was held or if saturated steam was present, both of which are critical for killing microorganisms.
H3: Biological Indicators Have a Lag Time
While biological indicators provide the most definitive proof of sterilization, they require an incubation period. This means the results are not available immediately, creating a delay before sterilized items can be confidently released for use.
How to Apply This to Your Protocol
A reliable sterilization protocol integrates all three indicators to compensate for their individual weaknesses. The right approach depends on the criticality of the items being sterilized.
- If your primary focus is routine, non-critical sterilization: Use physical indicators for every cycle and chemical indicator tape on every package to prevent mix-ups.
- If your primary focus is sterilizing critical items (e.g., medical devices, surgical tools): A combination of physical, chemical, and biological indicators is non-negotiable for every load.
- If your primary focus is quality control and validation: Regular biological indicator testing, combined with periodic calibration of the autoclave's physical indicators, is essential for protocol assurance.
Ultimately, a multi-faceted verification strategy is the only way to ensure absolute confidence in your sterilization process.
Summary Table:
| Indicator Type | What It Measures | Key Advantage | Key Limitation |
|---|---|---|---|
| Physical | Chamber temperature, pressure, and time | Real-time cycle monitoring | Cannot confirm conditions inside the load |
| Chemical | Exposure to specific temperature (e.g., via tape) | Immediate visual confirmation | Does not verify duration or steam penetration |
| Biological | Lethality against bacterial spores (e.g., Geobacillus stearothermophilus) | Gold standard for microbial kill | Requires incubation time for results |
Ensure your autoclave sterilization is fail-safe with KINTEK.
As your trusted partner in laboratory equipment and consumables, we provide the reliable indicators and validation tools you need to achieve and maintain sterility. Whether you're sterilizing routine labware or critical medical devices, our solutions help you build a robust, multi-layered verification protocol.
Contact us today to discuss your sterilization needs and let our experts help you select the right indicators for complete confidence in every cycle.
Get in touch with our specialists now →
Visual Guide
Related Products
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Desktop Fast Laboratory Autoclave Sterilizer 20L 24L for Lab Use
- Laboratory High Pressure Steam Sterilizer Vertical Autoclave for Lab Department
- Portable Digital Display Automatic Laboratory Sterilizer Lab Autoclave for Sterilization Pressure
- Portable High Pressure Laboratory Autoclave Steam Sterilizer for Lab Use
People Also Ask
- How much time is required for autoclave sterilization of instruments? Understand the Full Cycle for Safety
- What are the settings for autoclaving glassware? A Guide to Effective Sterilization
- How does an autoclave sterilize instruments supplies and equipment? A Guide to High-Pressure Steam Sterilization
- How do you sterilize glassware by autoclave? Master the 3-Step Process for Reliable Sterility
- What precautions should be taken when using an autoclave in the laboratory? A Guide to Safe Sterilization



















