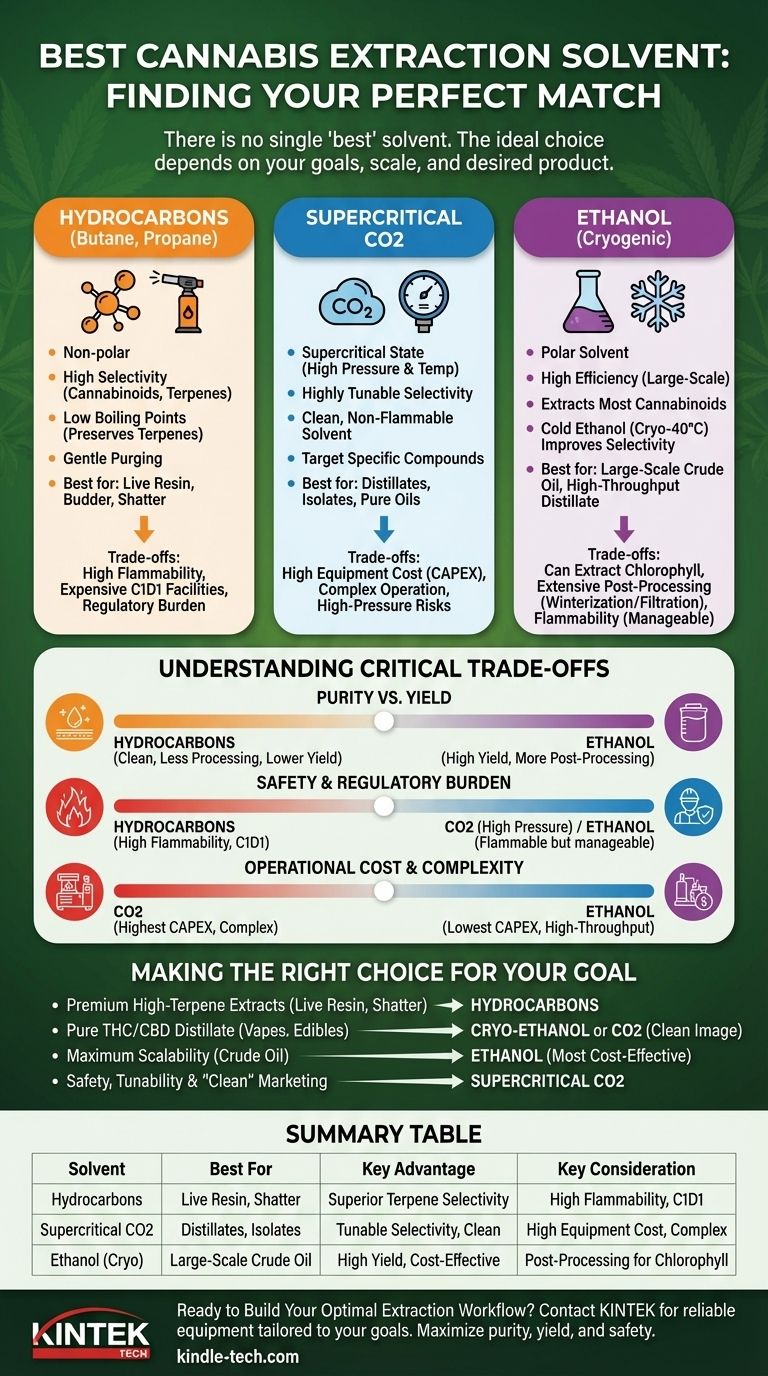
There is no single "best" solvent for cannabis extraction. The ideal choice is entirely dependent on your specific goals, as each primary solvent—hydrocarbons, CO2, and ethanol—offers a distinct profile of benefits and drawbacks. Selecting the right one requires a clear understanding of the desired end product, operational scale, safety requirements, and budget.
The choice of a solvent is a fundamental trade-off between selectivity (what you extract), efficiency (how much you extract), and safety (the operational risk). Your end product—whether it's a high-terpene live resin, a pure distillate, or a crude oil—will dictate which solvent is the most appropriate tool for the job.

The Three Primary Solvent Categories
To make an informed decision, you must first understand the fundamental properties of the three industry-standard solvents. Each interacts with the cannabis plant in a unique way, directly impacting the chemical profile of the final extract.
Hydrocarbons (Butane, Propane)
Hydrocarbons like butane and propane are non-polar solvents, renowned for their remarkable selectivity. They excel at dissolving cannabinoids (like THC and CBD) and terpenes while leaving behind undesirable water-soluble compounds like chlorophyll and plant metabolites.
This high selectivity is why hydrocarbons are the undisputed standard for producing high-aroma, high-terpene extracts such as live resin, budder, and shatter. The low boiling points of these solvents also allow for gentler purging processes, which helps preserve the delicate terpene profile.
Supercritical CO2
Carbon dioxide (CO2) becomes a solvent when it is brought to a supercritical state—a fluid phase achieved under specific high-pressure and high-temperature conditions. Its greatest advantage is its tunability.
By precisely adjusting the temperature and pressure, operators can fine-tune the solvent properties of CO2. This allows them to selectively target specific compounds, from light terpenes to heavier lipids. This makes CO2 extraction ideal for creating highly refined, pure oils destined for distillate and isolate production.
Ethanol
Ethanol is a highly efficient and powerful polar solvent. Its effectiveness at stripping cannabinoids from plant material makes it exceptionally well-suited for large-scale, high-throughput operations.
However, because ethanol is polar, it will also readily dissolve water-soluble compounds like chlorophyll if not managed carefully. To counteract this, modern ethanol extraction is almost always performed at cryogenic temperatures (e.g., -40°C). This "cold ethanol" method significantly improves selectivity, producing a high-quality crude oil that is perfect for large-scale distillate manufacturing.
Understanding the Critical Trade-offs
Choosing a solvent is an exercise in balancing competing priorities. What you gain in one area, you often sacrifice in another. Understanding these compromises is the key to making the right decision.
Purity vs. Yield
A highly selective solvent like butane will produce a very clean initial extract that requires less post-processing. However, this precision may leave some cannabinoids behind, slightly reducing the total yield.
Conversely, a highly efficient solvent like ethanol will extract nearly all available cannabinoids, resulting in a very high yield. This efficiency comes at the cost of also pulling unwanted compounds, which then must be removed through extensive post-processing steps like winterization and filtration.
Safety and Regulatory Burden
This is a non-negotiable factor. Hydrocarbons are extremely volatile and flammable, requiring expensive, explosion-proof environments (C1D1 facilities) and highly trained personnel. The regulatory burden is significant.
CO2 extraction operates under extremely high pressures, which poses a serious physical hazard if equipment fails. However, CO2 itself is non-flammable, making it a generally safer option than hydrocarbons from a fire-risk perspective.
Ethanol is flammable, but less so than hydrocarbons. While it still requires properly designed facilities and safety protocols, the associated risks and regulations are often considered more manageable, especially at a very large scale.
Operational Cost and Complexity
The initial capital expenditure (CAPEX) varies dramatically. Supercritical CO2 systems have the highest upfront cost due to the high-pressure equipment required.
Hydrocarbon systems have a moderate equipment cost, but the facility build-out for safety compliance can be extremely expensive.
Ethanol extraction systems generally have the lowest CAPEX, especially for large-scale setups, making it the most economically viable option for massive throughput operations.
Making the Right Choice for Your Goal
Your decision should be guided by the product you intend to create and the scale at which you will operate.
- If your primary focus is premium, high-terpene extracts (live resin, budder, shatter): Hydrocarbons are the undisputed industry standard due to their unparalleled selectivity for terpenes and cannabinoids.
- If your primary focus is producing pure THC/CBD distillate for vape cartridges or edibles: Cryo-ethanol is the leader for high-throughput efficiency, while CO2 is an excellent choice if a "clean solvent" marketing angle is a priority.
- If your primary focus is maximum scalability and efficiency for crude oil production: Ethanol is the most cost-effective and proven method for processing enormous volumes of biomass.
- If your primary focus is safety and tunability for specialized products: Supercritical CO2 offers a high degree of control and is often perceived as the "cleanest" extraction method.
Ultimately, understanding these trade-offs is the key to selecting the solvent that aligns perfectly with your operational goals and desired final product.
Summary Table:
| Solvent | Best For | Key Advantage | Key Consideration |
|---|---|---|---|
| Hydrocarbons (Butane/Propane) | Live Resin, Shatter, High-Terpene Extracts | Superior Terpene & Cannabinoid Selectivity | High Flammability, Requires C1D1 Facility |
| Supercritical CO2 | Distillates, Isolates, Pure Oils | Tunable Selectivity, Clean Solvent Image | High Equipment Cost, Complex Operation |
| Ethanol (Cryogenic) | Large-Scale Crude Oil, High-Throughput Distillate | High Yield & Cost-Effectiveness at Scale | Can Extract Chlorophyll (Requires Post-Processing) |
Ready to Build Your Optimal Extraction Workflow?
Choosing the right solvent is just the first step. You need reliable, high-performance equipment to execute your vision. KINTEK specializes in providing robust lab equipment and consumables tailored to the cannabis industry's unique demands.
We can help you select the right extraction and post-processing equipment to maximize purity, yield, and safety for your specific product goals—whether you're focusing on high-terpene extracts, pure distillates, or large-scale production.
Contact KINTEK today to discuss your project with our experts and discover how our solutions can enhance your lab's efficiency and product quality.
Visual Guide
Related Products
- Polygon Press Mold for Lab
- Ball Press Mold for Lab
- High-Purity Titanium Foil and Sheet for Industrial Applications
- Laboratory High Pressure Horizontal Autoclave Steam Sterilizer for Lab Use
- Molybdenum Disilicide (MoSi2) Thermal Elements Electric Furnace Heating Element
People Also Ask
- What is the duration of slow pyrolysis? A Deliberately Lengthy Process for Maximum Biochar Yield
- Is laser sintering the same as melting? Understand the Key Differences for Your AM Process
- What are the equipment for pyrolysis laboratory? Choosing the Right Reactor for Your Research
- What is pyrolysis equipment? Unlock the Value in Your Waste Materials
- What equipment is needed for pyrolysis? The 4 Core Components for a Successful Plant
- What is the high temperature of quartz? Key Thresholds for Crystalline vs. Fused Silica
- What is the history of hot isostatic pressing? A Deep Dive into High-Performance Densification
- What is the most efficient separation technique? Select the Best Method for Your Mixture

















