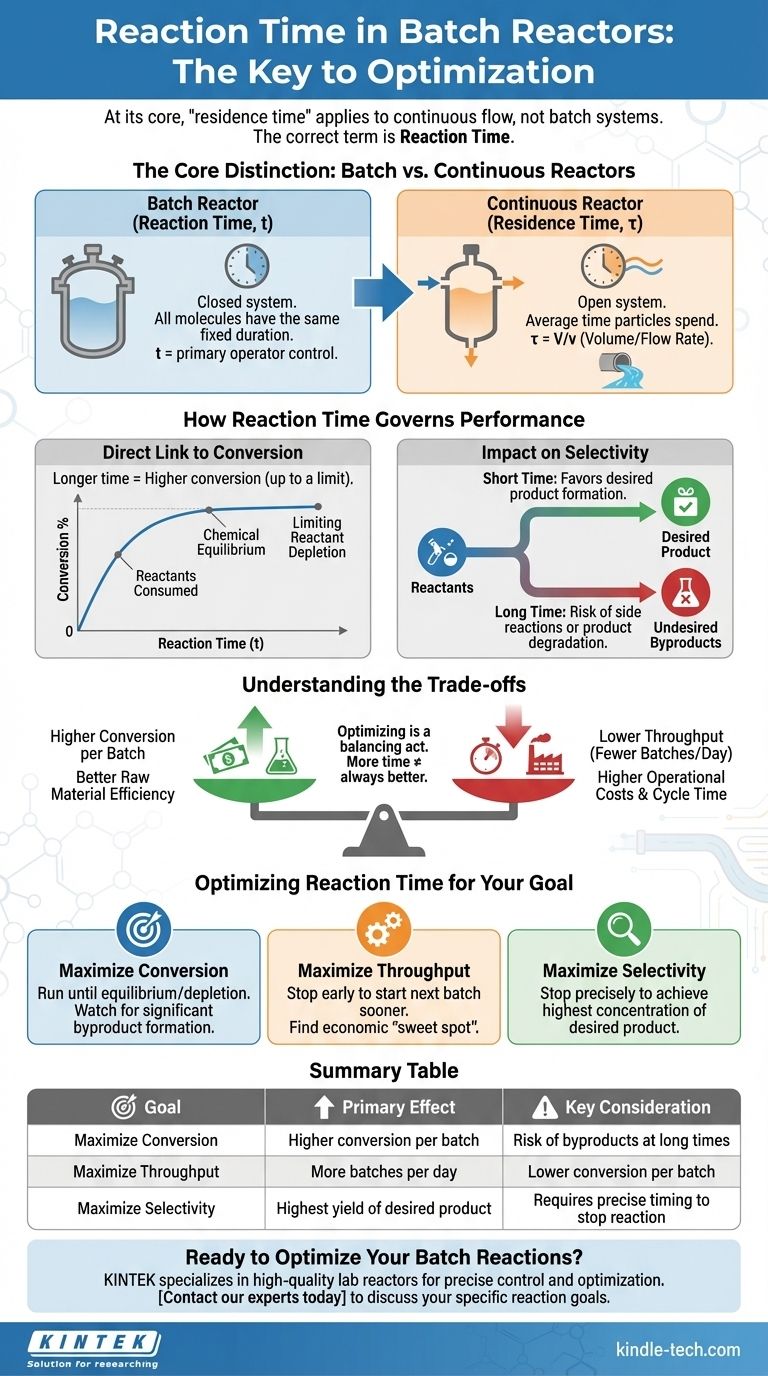
At its core, the concept of "residence time" does not directly apply to a batch reactor. This term is reserved for continuous flow systems. For a batch reactor, the equivalent and correct term is reaction time. A longer reaction time allows the reaction to proceed further, which generally increases the conversion of reactants into products, up until the point of chemical equilibrium or the complete depletion of a limiting reactant.
The critical distinction is that "residence time" describes the average duration a fluid element spends in a continuous flow reactor, while "reaction time" is the fixed duration for which all materials are held in a sealed batch reactor. This time is the primary operator-controlled variable for determining the final product conversion and yield.

The Core Distinction: Batch vs. Continuous Reactors
To understand the effect of time on a batch reaction, we must first clarify the terminology, as it reveals a fundamental difference in how these systems operate. This is not just semantics; it impacts process control, modeling, and optimization.
Why "Residence Time" Applies to Continuous Flow
Residence time (τ) is a concept for continuous reactors like a Continuous Stirred-Tank Reactor (CSTR) or a Plug Flow Reactor (PFR). In these systems, reactants constantly flow in and products constantly flow out.
Residence time is defined as the reactor volume (V) divided by the volumetric flow rate (v), or τ = V/v. It represents the average amount of time that a fluid particle spends inside the reactor. Some particles will exit faster than average, and some will stay longer.
The Batch Reactor Equivalent: "Reaction Time" (t)
A batch reactor is a closed system. All reactants are loaded into the vessel at the beginning (t=0), and the reaction proceeds for a set duration. Nothing is added or removed during this time.
The total time the reactants are allowed to react inside the vessel is called the reaction time (t). Unlike in a continuous reactor, every single molecule inside a batch reactor experiences the exact same reaction time. Think of it like baking a cake: all ingredients go in at once and are taken out together after a fixed baking time.
How Reaction Time Governs Batch Reactor Performance
Reaction time is the most direct lever you can pull to control the outcome of a batch process. By controlling how long you let the reaction run, you directly influence conversion, selectivity, and ultimately, your process economics.
The Direct Link to Conversion
For a given set of conditions (temperature, pressure, catalyst), the conversion of a reactant is a direct function of time. At the start (t=0), conversion is zero. As time progresses, reactants are consumed, and conversion increases.
This relationship is described by the reaction's rate law. A longer reaction time allows the reaction to proceed further along its kinetic path, resulting in a higher concentration of products and a lower concentration of remaining reactants.
Reaching Equilibrium or Full Conversion
The increase in conversion with time is not infinite. The reaction will stop progressing for one of two reasons:
- Limiting Reactant Depletion: One of the reactants is completely consumed, achieving 100% conversion for that reactant.
- Chemical Equilibrium: For reversible reactions, the reaction will proceed until it reaches a state of dynamic equilibrium, where the forward reaction rate equals the reverse reaction rate. At this point, the net conversion will no longer change with additional time.
Impact on Selectivity and Side Reactions
In many industrial processes, multiple reactions can occur simultaneously. Selectivity measures how much of the converted reactant forms the desired product versus undesired byproducts.
Reaction time is a critical tool for controlling selectivity. A short reaction time might favor the formation of the desired product, while a longer time could allow for slower, undesired side reactions to occur or for the desired product itself to degrade into something else.
Understanding the Trade-offs of Reaction Time
Optimizing a batch reactor is a balancing act. Simply running the reaction for as long as possible is rarely the best economic strategy. You must weigh the benefits of higher conversion against several significant costs.
The Pursuit of Higher Conversion
The primary benefit of a longer reaction time is higher conversion per batch. This means you get more product from the same amount of starting material, which can improve raw material efficiency.
The Cost of Throughput
The most significant trade-off is throughput, or the total amount of product you can make in a given operational period (e.g., per day).
Every batch cycle includes time for filling, heating, reacting, cooling, and emptying. A longer reaction time directly increases the total cycle time. This means you can run fewer batches per day. An optimal process often involves stopping the reaction before it's complete to start the next batch sooner, maximizing the overall production rate.
The Risk of Undesired Byproducts
As mentioned, excessive reaction time can harm selectivity. If the value of the desired product is high and the byproducts are waste, over-reacting can decrease the profitability of the batch even if total reactant conversion is high.
Energy and Operational Costs
Longer reaction times mean longer operational cycles. This translates directly to higher utility costs for maintaining reaction temperature (heating or cooling), running mixers, and occupying equipment that could be used for another batch.
Optimizing Reaction Time for Your Goal
The "best" reaction time is not a single number; it depends entirely on your primary business or operational objective.
- If your primary focus is maximizing conversion per batch: Increase reaction time until you approach chemical equilibrium or until a key reactant is fully depleted, but monitor for significant byproduct formation.
- If your primary focus is maximizing plant throughput (e.g., tons per day): Find the economic sweet spot where the marginal gain in conversion from extending the reaction time is outweighed by the cost of a longer cycle time. This often means stopping the reaction well before it reaches maximum conversion.
- If your primary focus is maximizing selectivity: Carefully determine the optimal time to stop the reaction to achieve the highest possible concentration of your desired product before it begins to degrade or convert into byproducts.
Ultimately, mastering reaction time is key to controlling the delicate balance between product quality, production rate, and operational cost in any batch process.
Summary Table:
| Reaction Time Goal | Primary Effect | Key Consideration |
|---|---|---|
| Maximize Conversion | Higher conversion per batch | Risk of byproducts at long times |
| Maximize Throughput | More batches per day | Lower conversion per batch |
| Maximize Selectivity | Highest yield of desired product | Requires precise timing to stop reaction |
Ready to Optimize Your Batch Reactions?
Mastering reaction time is key to balancing product quality, production rate, and cost. KINTEK specializes in providing high-quality lab reactors and consumables, empowering you to precisely control and optimize your batch processes for maximum efficiency and yield.
Contact our experts today to discuss how our equipment can help you achieve your specific reaction goals.
Visual Guide
Related Products
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
People Also Ask
- Why is a glass reactor under nitrogen protection used for PDMS-b-PCL synthesis? Ensure Purity & Precise Polymerization
- How do high-performance photocatalytic reactors ensure data reliability? Master AQY with Precision Light & Thermal Control
- What is the function of a high-pressure stainless steel autoclave in the catalytic conversion of cellulose into sugar alcohols?
- What is the role of a high-pressure reactor in Fenton catalysts? Engineer High-Activity Spinel Ferrites with Precision
- What are the technical advantages of deionized water in supercritical CFRP decomposition? Efficient & Sustainable.
- What is the mode of operation of a batch reactor? A Step-by-Step Guide to Its Flexible Process
- Why is a high-pressure reactor necessary for the acid hydrolysis process? Optimize PLA Bioplastic Production
- Why glass lined reactors are used? Achieve Unmatched Purity & Corrosion Resistance



















