When selecting an autoclave, your decision must be driven by three core factors: the types of materials you need to sterilize, the required speed and throughput of your workflow, and the non-negotiable safety features that protect your personnel and facility. Considering chamber size, sterilization method (gravity vs. vacuum), and control systems will ensure you invest in a unit that is both effective and appropriate for your specific application.
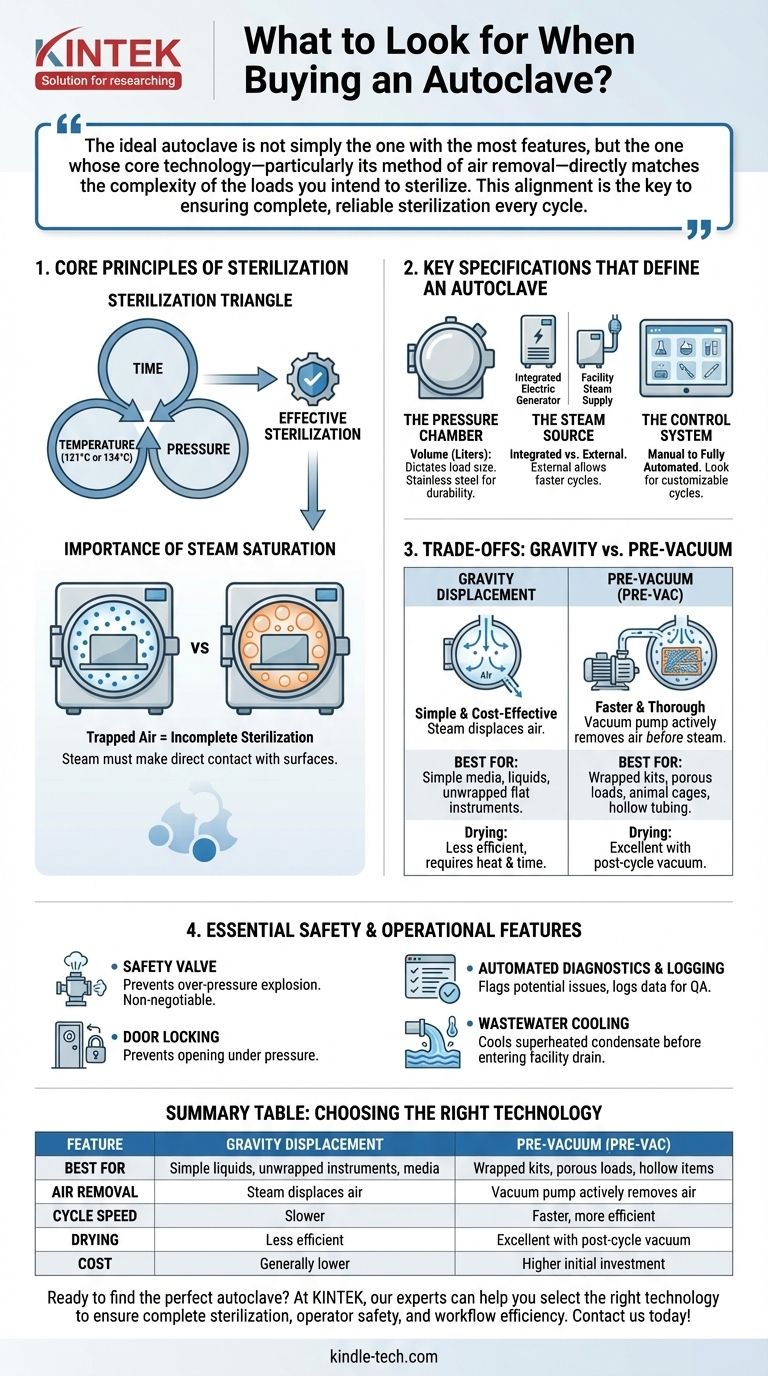
The ideal autoclave is not simply the one with the most features, but the one whose core technology—particularly its method of air removal—directly matches the complexity of the loads you intend to sterilize. This alignment is the key to ensuring complete, reliable sterilization every cycle.

First, Understand the Core Principles of Sterilization
To choose the right tool, you must first understand the job it performs. An autoclave's function is elegantly simple: to use high-pressure, saturated steam to kill all microorganisms.
The Sterilization Triangle: Time, Temperature, and Pressure
Effective sterilization hinges on a precise relationship between temperature, pressure, and time. Steam under pressure can reach temperatures far above the boiling point of water (typically 121°C or 134°C), which is necessary to denature the proteins within microorganisms. The control system must hold these conditions for a specific duration to ensure a complete kill.
The Importance of Steam Saturation
The enemy of effective sterilization is trapped air. Air pockets act as an insulating barrier, preventing steam from making direct contact with surfaces. The primary difference between autoclave types lies in how they solve this problem by removing air from the chamber.
Key Specifications That Define an Autoclave
Your evaluation should begin with the fundamental hardware and its capabilities. These components dictate the autoclave's capacity, speed, and suitability for your lab.
The Pressure Chamber: Size and Construction
The chamber is the heart of the autoclave. Its volume (measured in liters) dictates the size and quantity of the load you can process in a single cycle. The inner chamber and outer jacket are typically made of stainless steel to withstand repeated high-pressure, high-temperature cycles.
The Steam Source: Integrated vs. External
Autoclaves are powered by steam. Some units have a built-in electric steam generator that heats water within a reservoir, making them self-contained. Others are designed to connect to a central, in-house steam supply, which can support faster cycles and higher throughput.
The Control System: Manual to Fully Automated
Modern autoclaves feature sophisticated control panels and software. Look for systems that offer pre-programmed cycles for different load types (e.g., glassware, liquids, instruments) as well as the ability to customize cycle parameters (time, temperature, drying).
Understanding the Trade-offs: Gravity vs. Pre-Vacuum
The single most important technical decision is choosing the method of air removal. This choice directly impacts what you can and cannot reliably sterilize.
Gravity Displacement: Simple and Cost-Effective
In a gravity autoclave, steam is introduced into the top of the chamber. Because steam is less dense than air, it displaces the heavier air, forcing it out through a drain at the bottom. This method is simple and reliable for sterilizing uncomplicated loads like lab media, water, and unwrapped flat instruments.
Pre-Vacuum (Pre-Vac): Faster and More Thorough
A pre-vacuum (or pre-vac) autoclave uses a vacuum pump to actively remove air from the chamber before steam is introduced. This is often done in a series of pulses. By creating a near-vacuum, steam can instantly penetrate dense, porous, or complex loads like wrapped instrument kits, animal cages, textiles, and hollow tubing.
The Critical Role of Drying
Effective sterilization ends with a dry load. Pre-vac autoclaves excel here, as they typically include a post-cycle vacuum phase that boils away residual moisture, leaving instruments ready for immediate use or storage. Gravity units rely on heat and a longer wait time, which is less efficient.
Essential Safety and Operational Features
An autoclave is a high-pressure vessel, and safety cannot be an afterthought. Scrutinize these features to ensure operational integrity.
The Safety Valve: Your Fail-Safe Against Over-Pressure
Every autoclave is equipped with a safety valve. Its sole purpose is to prevent a catastrophic failure (an explosion) by automatically venting steam if the pressure inside the chamber exceeds a safe limit. This is a critical, non-negotiable safety mechanism, not a danger in itself.
Door Locking Mechanisms
The door must remain securely locked under pressure. Modern autoclaves have safety interlocks that prevent the door from being opened until the chamber has returned to a safe temperature and pressure.
Automated Diagnostics and Cycle Logging
Advanced units include self-diagnostic systems that can flag potential issues before they cause a cycle failure. The ability to log cycle data is also crucial for quality assurance and compliance in many clinical and research settings.
Wastewater Cooling
The superheated condensate discharged at the end of a cycle can damage plumbing. Many autoclaves include a system to cool this wastewater before it enters the facility drain, protecting your infrastructure.
Making the Right Choice for Your Application
Use your primary sterilization tasks as your guide to select the right technology.
- If your primary focus is sterilizing simple media, liquids, and flat glassware: A gravity displacement autoclave is likely sufficient and more cost-effective.
- If your primary focus is sterilizing porous loads, wrapped instruments, or hollow tubing: A pre-vacuum autoclave is essential for ensuring complete steam penetration and effective sterilization.
- If your primary focus is high-throughput and rapid turnaround: Prioritize a pre-vacuum model with an efficient post-vacuum drying cycle and, if available, a direct facility steam source.
By matching the autoclave's core technology to your specific sterilization needs, you ensure both process integrity and operator safety.
Summary Table:
| Feature | Gravity Displacement | Pre-Vacuum (Pre-Vac) |
|---|---|---|
| Best For | Simple liquids, unwrapped instruments, media | Wrapped kits, porous loads, hollow items |
| Air Removal | Steam displaces air | Vacuum pump actively removes air |
| Cycle Speed | Slower | Faster, more efficient |
| Drying | Less efficient | Excellent with post-cycle vacuum |
| Cost | Generally lower | Higher initial investment |
Ready to find the perfect autoclave for your lab's specific needs?
At KINTEK, we specialize in providing high-quality lab equipment, including autoclaves tailored for your sterilization challenges. Whether you need a cost-effective gravity displacement model for simple media or a high-throughput pre-vacuum system for complex loads, our experts can help you select the right technology to ensure complete sterilization, operator safety, and workflow efficiency.
Contact us today to discuss your requirements and let KINTEK enhance your laboratory's capabilities. Get in touch now!
Visual Guide
Related Products
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Test Sieves and Sieving Machines
- Benchtop Laboratory Vacuum Freeze Dryer
People Also Ask
- What are the standard operating parameters for an autoclave? Master Temperature, Pressure, and Time for Sterilization
- What is the function of laboratory autoclaves in SCWR research? Predict Material Compatibility and Corrosion Kinetics
- What is the primary function and principle of autoclaving? Master Lab Sterilization with High-Pressure Steam
- What critical environmental conditions does a laboratory autoclave provide for evaluating wear resistance? - KINTEK
- What role do laboratory autoclaves play in pectin extraction? Optimize Prebiotic Yield from Citrus and Apple Biomass



















