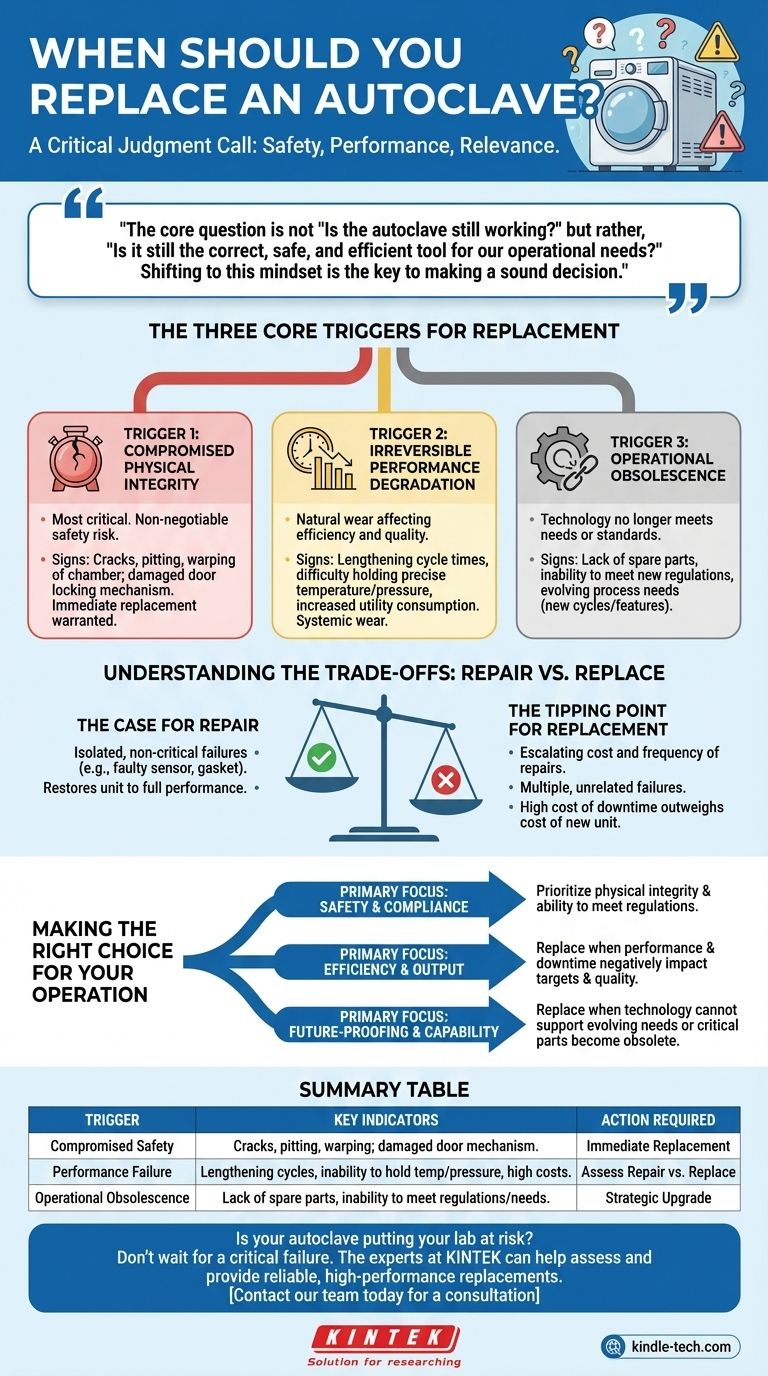
Deciding when to replace an autoclave is a critical judgment call based on three primary factors. It is not about a fixed age or timeline, but rather a clear-eyed assessment of its safety, performance, and relevance. The decision to replace is triggered when the equipment's physical integrity is compromised, its performance consistently fails to meet standards, or it becomes technologically obsolete.
The core question is not "Is the autoclave still working?" but rather, "Is it still the correct, safe, and efficient tool for our operational needs?" Shifting to this mindset is the key to making a sound decision.

The Three Core Triggers for Replacement
Understanding the specific signals that indicate a need for replacement allows you to move from a reactive "fix-it" approach to a proactive management strategy for this critical asset.
Trigger 1: Compromised Physical Integrity
This is the most critical and non-negotiable trigger for replacement. It centers on the core safety of the pressure vessel and its associated components.
Minor parts like gaskets or valves can often be repaired, but damage to fundamental systems warrants immediate consideration for full replacement. Signs of compromised integrity include any cracks, pitting, or warping of the chamber, or damage to the door locking mechanism.
An autoclave is a high-pressure environment. Continuing to operate a unit with questionable structural integrity creates an unacceptable risk of catastrophic failure and serious injury.
Trigger 2: Irreversible Performance Degradation
This trigger relates to the natural wear and tear that impacts efficiency and product quality. Over time, an autoclave may no longer operate at its best.
Key indicators include lengthening cycle times, difficulty holding precise temperatures or pressures, and increased utility consumption. These issues point to a decline in the unit's core efficiency.
While a single faulty sensor can be replaced, a pattern of declining performance suggests systemic wear that repairs can no longer effectively address, leading to inconsistent sterilization and higher operational costs.
Trigger 3: Operational Obsolescence
An autoclave can be in perfect working condition but still be the wrong tool for the job. Obsolescence occurs when the technology no longer meets current needs or standards.
This can be driven by a lack of available spare parts for an older model, making repairs impossible. It may also be due to an inability to meet new regulatory requirements, such as advanced data logging for compliance.
Furthermore, your operational needs may evolve. If you require new cycles or features that your current unit cannot support, it has become obsolete for your purposes, regardless of its mechanical condition.
Understanding the Trade-offs: Repair vs. Replace
Every decision involves balancing cost, downtime, and risk. It's crucial to identify the tipping point where frequent repairs become more costly than a strategic replacement.
The Case for Repair
Repair is the logical choice for isolated, non-critical component failures. Replacing a faulty temperature probe, a worn door gasket, or a malfunctioning solenoid is routine maintenance.
The key is that the repair restores the unit to its full, expected performance without compromising the integrity of the larger system.
The Tipping Point for Replacement
Replacement becomes the superior option when the cost and frequency of repairs begin to escalate. If you are experiencing multiple, unrelated failures in a short period, it signals the end of the unit's reliable service life.
The most significant factor is the cost of downtime. If a failing autoclave is constantly halting production or research, the financial impact of that lost time can easily outweigh the cost of a new, reliable unit.
Making the Right Choice for Your Operation
Use your primary operational driver to guide your final decision.
- If your primary focus is safety and compliance: Prioritize the physical integrity of the chamber and its ability to meet all current regulations.
- If your primary focus is efficiency and output: Replace the unit when performance degradation and downtime begin to negatively impact your operational targets and quality.
- If your primary focus is future-proofing and capability: Replace the unit when its technology can no longer support your evolving needs or when critical parts become obsolete.
Proactively managing your autoclave's lifecycle is an investment in safety, efficiency, and operational continuity.
Summary Table:
| Replacement Trigger | Key Indicators | Action Required |
|---|---|---|
| Compromised Safety | Cracks, pitting, or warping in the chamber; damaged door mechanism. | Immediate Replacement |
| Performance Failure | Lengthening cycles, inability to hold temperature/pressure, high utility costs. | Assess Repair vs. Replace |
| Operational Obsolescence | Lack of spare parts, inability to meet new regulations or process needs. | Strategic Upgrade |
Is your autoclave putting your lab at risk? Don't wait for a critical failure. The experts at KINTEK can help you assess your equipment's condition and provide a reliable, high-performance replacement tailored to your laboratory's specific needs in sterilization and safety.
Contact our team today for a consultation to ensure your operations remain safe, efficient, and compliant.
Visual Guide
Related Products
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Test Sieves and Sieving Machines
- Benchtop Laboratory Vacuum Freeze Dryer
People Also Ask
- What is an autoclave laboratory equipment? The Ultimate Guide to Steam Sterilization
- How long are autoclaved items sterile? Understanding Event-Related Sterility for Lab Safety
- Why are high-temperature and high-pressure reactors (autoclaves) essential for friction and wear tests? Get Real Data
- Why is the autoclave process considered ideal for manufacturing fiber-reinforced laminates containing self-healing elements?
- Is autoclave suitable for all materials? The Definitive Guide to Safe Sterilization
- What role does a laboratory autoclave play in HEA corrosion research? Key to Validating Advanced Reactor Materials
- Is a retort a pressure vessel? The Critical Safety Classification for Your Sterilization Process
- What is the efficiency of an autoclave? Achieving Total Sterilization with High-Pressure Steam



















