Strictly speaking, evaporation doesn't need a vacuum. However, for highly technical and industrial processes, creating a vacuum is essential for controlling the process. It solves two distinct problems: it dramatically lowers the boiling point of liquids and it removes atmospheric gases that interfere with vapor particles traveling from a source to a target.
The core reason for using a vacuum in evaporation is to gain precise control over a material's state change. A vacuum removes atmospheric pressure, which either allows liquids to boil at much lower, safer temperatures or ensures vaporized particles can travel unimpeded to form a pure, high-quality coating.

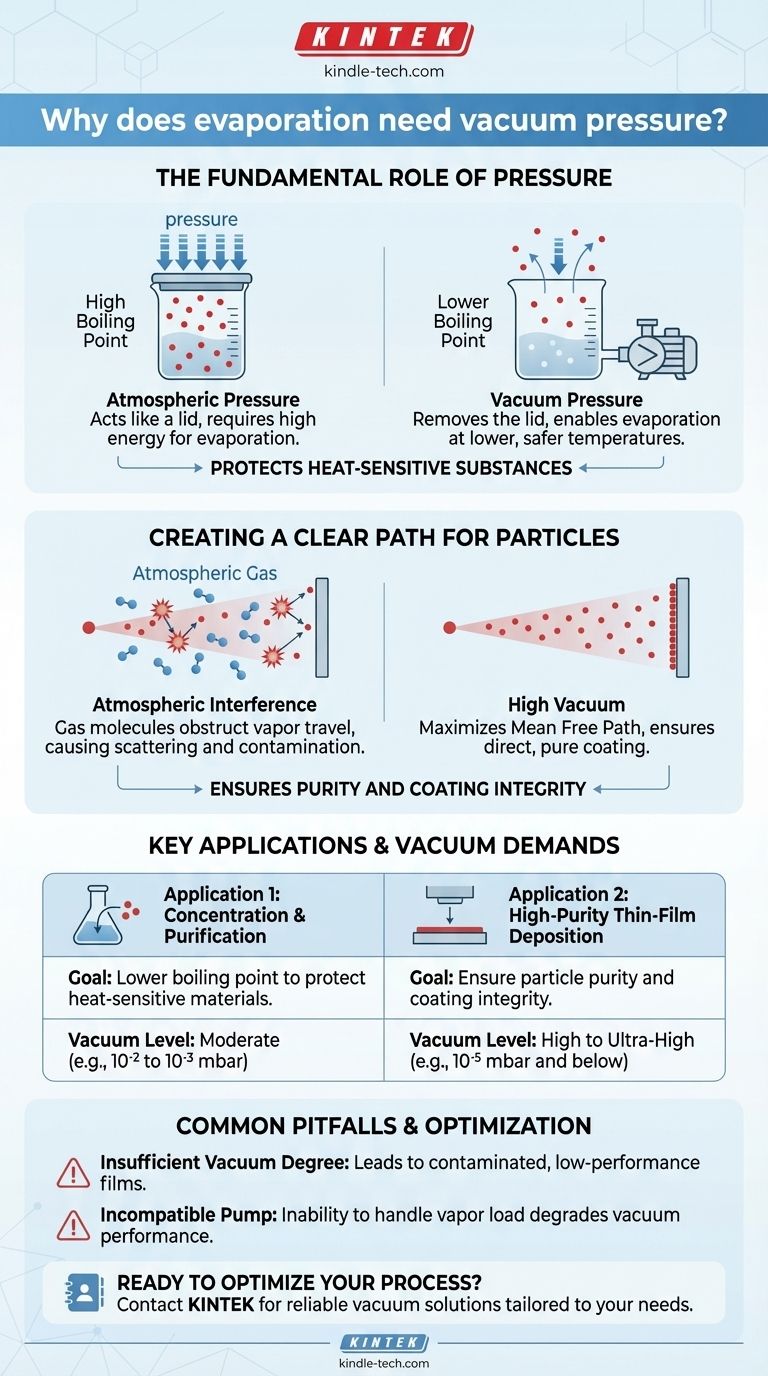
The Fundamental Role of Pressure
To understand the need for a vacuum, we must first understand the role of the air around us. The atmosphere exerts constant pressure on everything, including the surface of liquids.
Lowering the Boiling Point
Atmospheric pressure acts like a lid on a pot, making it harder for liquid molecules to escape and turn into a gas.
By using a vacuum pump to remove the air, we effectively remove that lid. With less pressure holding them down, liquid molecules can escape into a vapor phase using far less energy—meaning, at a much lower temperature.
This principle is critical in processes like rotary evaporation or wastewater treatment, where the goal is to evaporate a solvent (like water) without damaging or degrading the temperature-sensitive substance dissolved within it.
Creating a Clear Path for Particles
In other applications, like physical vapor deposition (PVD), the goal isn't to boil a bulk liquid but to deposit a microscopic, high-purity film onto a surface (a substrate).
In this context, the air molecules between the evaporation source and the substrate are obstacles. Vaporized material particles will collide with nitrogen, oxygen, and other gas molecules, scattering them and preventing them from reaching the target cleanly.
Worse, these atmospheric gases can react with the vapor, introducing impurities that contaminate the final film and degrade its quality. A high vacuum (like 10⁻⁶ Torr) creates an extremely long mean free path—the average distance a particle can travel before hitting something else—ensuring a direct, uninterrupted journey to the substrate.
Key Applications and Their Vacuum Demands
The reason for the vacuum dictates the level of vacuum required. The two primary applications demonstrate this difference clearly.
Application 1: Concentration and Purification
In this scenario, the goal is to separate a liquid solvent from a dissolved solid or a less volatile liquid. This is common in chemical purification and wastewater concentration.
Here, the vacuum's primary job is to lower the boiling point. A relatively low-grade vacuum is often sufficient to achieve the desired temperature reduction, protecting the integrity of the target substance.
Application 2: High-Purity Thin-Film Deposition
This process is used to create components for electronics, optics, and medical devices, such as the layers in an OLED screen.
The primary goals are purity and structural integrity. The vacuum must remove virtually all background gas molecules to prevent scattering and contamination. This requires a high or ultra-high vacuum (pressures of 10⁻⁵ mbar or lower) to guarantee the deposited atoms arrive uncontaminated and form a stable, high-quality coating.
Common Pitfalls and Considerations
Simply applying a vacuum is not enough; the quality and stability of that vacuum are what determine the success of the process.
The Degree of Vacuum is Crucial
The required vacuum level is directly tied to the desired outcome. Using a vacuum that is insufficient for a PVD process will result in a contaminated, low-performance film. A vacuum of 6 x 10⁻² Pa might be a minimum starting point, but high-tech applications demand pressures many orders of magnitude lower.
Not All Pumps Are Equal
The vacuum pump must be capable of handling the condensable vapors it is helping to create. If the pump cannot manage this vapor load, its own performance will degrade, causing the vacuum level to drop and compromising the entire process.
How to Apply This to Your Goal
The right approach depends entirely on what you are trying to achieve with evaporation.
- If your primary focus is separating a heat-sensitive compound from a solvent: Your goal is to lower the boiling point, so a pump that achieves a stable, moderate vacuum is your most effective tool.
- If your primary focus is depositing a high-purity coating: Your goal is to maximize the mean free path and eliminate contaminants, requiring a high-vacuum system designed for purity.
Understanding these core principles allows you to move beyond simply using a vacuum and begin wielding it as a precision tool for material control.
Summary Table:
| Application | Primary Goal | Required Vacuum Level |
|---|---|---|
| Concentration & Purification | Lower boiling point to protect heat-sensitive materials | Moderate (e.g., 10⁻² to 10⁻³ mbar) |
| Thin-Film Deposition (PVD) | Ensure particle purity and coating integrity | High to Ultra-High (e.g., 10⁻⁵ mbar and below) |
Ready to Optimize Your Evaporation Process?
Whether you are concentrating heat-sensitive compounds or depositing high-purity coatings, the right vacuum system is critical to your success. KINTEK specializes in lab equipment and consumables, providing reliable solutions tailored to your laboratory's specific needs.
Our experts can help you select the ideal vacuum pump and system to ensure precise temperature control, eliminate contamination, and achieve superior results in your applications.
Contact KINTEK today to discuss your requirements and discover how our expertise can enhance your lab's efficiency and output quality.
Visual Guide
Related Products
- Vacuum Hot Press Furnace Heated Vacuum Press Machine Tube Furnace
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
People Also Ask
- In what ways does a vacuum hot pressing furnace enhance AMCs? Achieve Near-Theoretical Density and Strength
- What is pressure sintering? Achieve High-Density Materials Faster and Stronger
- Why is it essential to maintain a high vacuum state during hot press sintering? Optimize SiCp/2024Al Quality
- Why are specialized environmental control units necessary for micro-scale testing? Protect Your Data Integrity
- How does the vacuum environment within a hot pressing furnace protect SiCf/Ti-43Al-9V? Ensure Composite Integrity



















