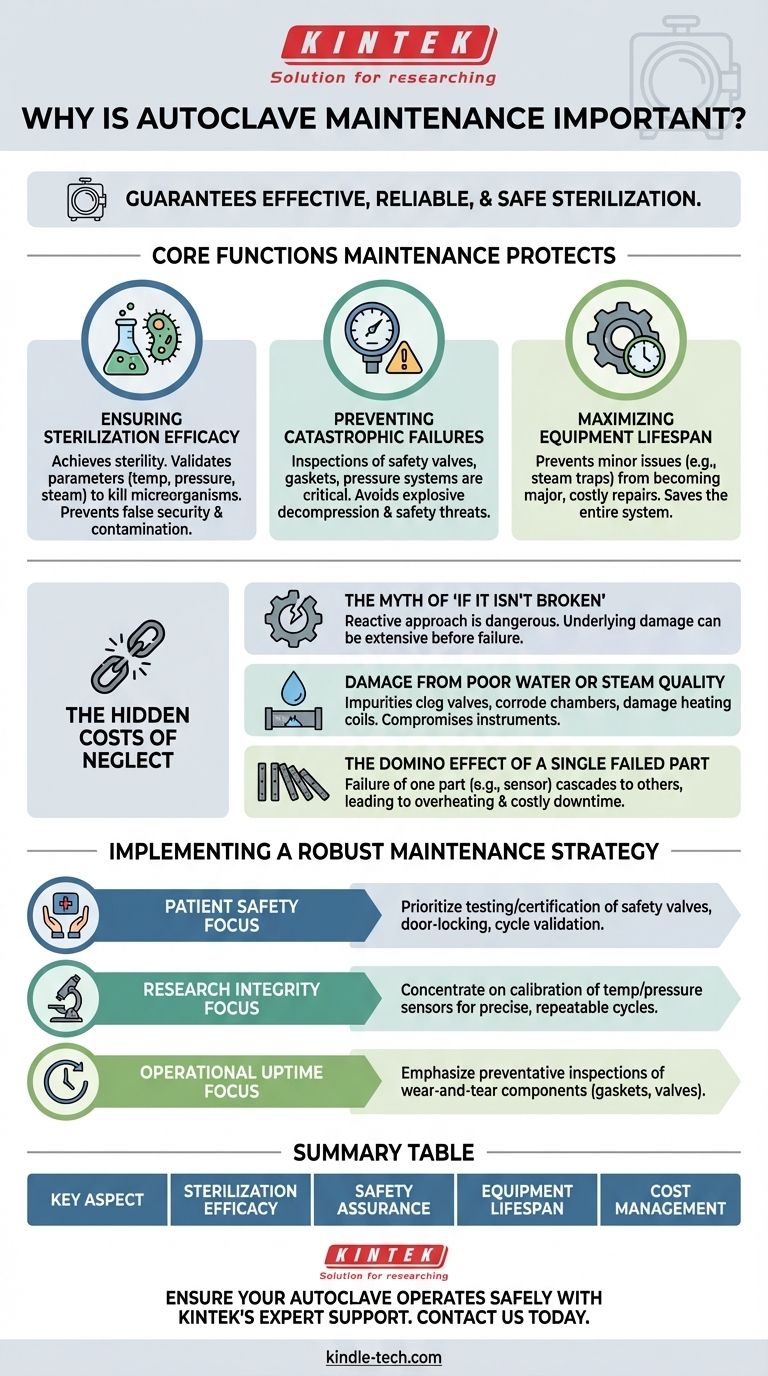
In short, autoclave maintenance is essential because it guarantees the sterilization process is effective, reliable, and safe. Without it, you cannot be certain that instruments are sterile, putting patient safety or experimental integrity at severe risk and creating conditions for catastrophic equipment failure.
The core purpose of autoclave maintenance isn't just to prevent breakdowns; it's to validate that every single sterilization cycle performs its critical function correctly. It transforms the autoclave from an unpredictable liability into a scientifically reliable instrument.

The Core Functions Maintenance Protects
Proper maintenance underpins the very reason an autoclave exists. Neglecting it fundamentally compromises its function in several critical areas.
Ensuring Sterilization Efficacy
The primary goal of an autoclave is to achieve sterility. Routine maintenance ensures that temperature, pressure, and steam penetration meet the precise parameters required to kill all microorganisms.
A poorly maintained machine may complete a cycle, but it might not reach the necessary conditions, resulting in a false sense of security and potentially contaminated materials.
Preventing Catastrophic Failures
Autoclaves are pressurized vessels operating at high temperatures. They are inherently dangerous if not properly maintained.
Regular inspections of safety valves, door gaskets, and pressure systems are critical safety procedures. A failure in these components can lead to explosive decompression, posing a significant threat to personnel and property.
Maximizing Equipment Lifespan
Consistent maintenance prevents minor issues from escalating into major, costly repairs. Problems often start with small, inexpensive components.
For example, a malfunctioning steam trap can lead to poor steam quality, which in turn can damage the entire chamber and the items being sterilized. Addressing the trap early saves the system.
The Hidden Costs of Neglect
Viewing maintenance as an optional expense is a critical error. The costs of deferring essential upkeep are far greater than the costs of a scheduled maintenance plan.
The Myth of "If It Isn't Broken"
A reactive approach to autoclave maintenance is dangerous. By the time a problem becomes obvious—such as a failed cycle or a visible leak—the underlying damage may already be extensive and the integrity of previous cycles is thrown into question.
Damage from Poor Water or Steam Quality
The references correctly identify that particulates in steam or poor water quality are a primary source of damage. These impurities can clog valves, corrode the chamber, and damage heating coils.
This not only shortens the life of the machine but can also leave deposits on sterilized instruments, compromising their use.
The Domino Effect of a Single Failed Part
An autoclave is an interconnected system. The failure of one part, like a sensor or contactor, places stress on others, leading to a cascade of failures.
What begins as a simple electrical fault can quickly lead to overheating, pressure irregularities, and complete system shutdown, causing costly downtime.
Implementing a Robust Maintenance Strategy
A proactive maintenance schedule is the only way to ensure safety, efficacy, and reliability. Your specific focus will determine your priorities.
- If your primary focus is patient safety: Prioritize the regular testing and certification of safety valves, door-locking mechanisms, and cycle validation with biological indicators.
- If your primary focus is research integrity: Concentrate on the calibration of temperature and pressure sensors to guarantee cycle parameters are precise and repeatable.
- If your primary focus is operational uptime: Emphasize preventative inspections of wear-and-tear components like gaskets, valves, and steam traps to avoid unexpected downtime.
Ultimately, consistent autoclave maintenance is the foundation of a safe and effective sterilization program.
Summary Table:
| Key Aspect of Maintenance | Why It Matters |
|---|---|
| Sterilization Efficacy | Ensures temperature, pressure, and steam meet parameters to kill microorganisms. |
| Safety Assurance | Prevents catastrophic failures like explosive decompression through valve and gasket checks. |
| Equipment Lifespan | Maximizes longevity by addressing minor issues (e.g., steam traps) before they escalate. |
| Cost Management | Avoids hidden costs of neglect, such as downtime, repairs, and compromised cycles. |
Ensure your autoclave operates safely and effectively with KINTEK's expert support. Whether you're focused on patient safety, research integrity, or operational uptime, our lab equipment solutions—including maintenance services, consumables, and calibration—deliver reliability and peace of mind. Don't risk sterilization failures; contact us today to schedule a consultation or learn how we can optimize your autoclave performance!
Visual Guide
Related Products
- Portable High Pressure Laboratory Autoclave Steam Sterilizer for Lab Use
- Laboratory Sterilizer Lab Autoclave Herbal Powder Sterilization Machine for Plant
- Laboratory Sterilizer Lab Autoclave Pulsating Vacuum Desktop Steam Sterilizer
- Laboratory High Pressure Steam Sterilizer Vertical Autoclave for Lab Department
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
People Also Ask
- Why is a laboratory high-pressure autoclave sterilizer necessary? Ensure Accuracy in Antibacterial Research
- What role does a laboratory autoclave play in the separation of lignin? High-Purity Extraction for Biomass Research
- What is the function of laboratory autoclaves in SCWR research? Predict Material Compatibility and Corrosion Kinetics
- What is the primary function and principle of autoclaving? Master Lab Sterilization with High-Pressure Steam
- What are the standard operating parameters for an autoclave? Master Temperature, Pressure, and Time for Sterilization



















