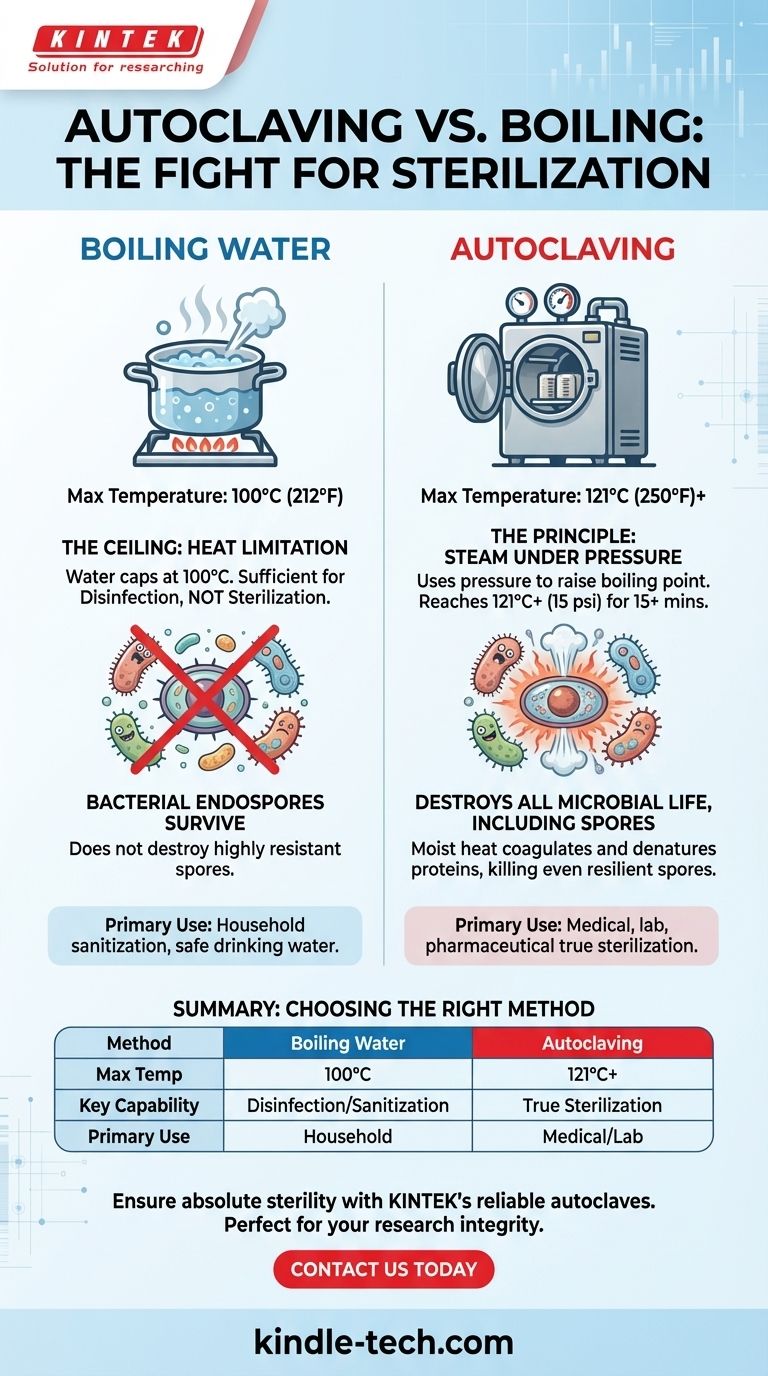
The fundamental reason autoclaving is used over boiling is that it achieves a significantly higher temperature, which is essential for true sterilization. While boiling water is capped at 100°C (212°F), an autoclave uses steam under pressure to reach 121°C (250°F) or higher, a temperature required to destroy the most resilient forms of microbial life.
The critical difference is not just heat, but the quality of heat. Autoclaving uses pressurized steam to exceed water's normal boiling point, providing the thermal energy necessary to reliably kill heat-resistant bacterial spores that can easily survive a full boil.

The Physical Limitation of Boiling Water
To understand why autoclaving is the standard for sterilization, we must first recognize the inherent ceiling of boiling water.
The 100°C Temperature Ceiling
At standard atmospheric pressure, water boils at 100°C (212°F). No matter how long you boil it or how vigorously, the temperature of the water itself will not increase beyond this point.
This temperature is sufficient for disinfection—killing many active bacteria, viruses, and fungi. However, it is not sufficient for sterilization.
The Challenge of Bacterial Endospores
The primary obstacle to sterilization is the bacterial endospore. These are dormant, highly resistant structures produced by certain bacteria as a survival mechanism.
Spores can withstand extreme conditions, including boiling water, radiation, and harsh chemicals. To achieve true sterilization, a process must be able to verifiably destroy these resilient spores. Boiling water simply does not get hot enough to do this reliably.
How Autoclaving Achieves True Sterilization
An autoclave is essentially a sophisticated pressure cooker. It leverages a fundamental law of physics to overcome the limitations of simple boiling.
The Principle: Steam Under Pressure
By increasing the pressure inside a sealed chamber, an autoclave raises the boiling point of water. This allows the steam inside to reach much higher temperatures.
The standard for medical and laboratory sterilization is 121°C (250°F), achieved at a pressure of approximately 15 pounds per square inch (psi) for a minimum of 15 minutes.
The Power of Moist Heat
The high-temperature steam generated in an autoclave is exceptionally effective at transferring thermal energy. This moist heat rapidly penetrates materials and kills microorganisms.
It works by coagulating and denaturing essential proteins and enzymes within the microbial cells, including the tough protective layers of endospores. This process is far more efficient than dry heat at the same temperature.
Understanding the Trade-offs
While autoclaving is the gold standard, it's important to recognize that it is not always necessary or practical. The choice between methods depends entirely on the desired outcome.
When Boiling Is "Good Enough"
Boiling is a form of sanitization or disinfection, not sterilization. It is perfectly adequate for many household applications, such as making water safe to drink or sanitizing baby bottles.
The goal in these cases is to reduce the number of active pathogens to a safe level, not to eliminate all microbial life.
The Requirements of Autoclaving
Autoclaving is an industrial-grade process. It requires specialized, validated equipment and trained operators. Furthermore, the high heat and pressure can damage certain materials, such as heat-sensitive plastics or delicate electronics.
This method is reserved for environments where absolute sterility is non-negotiable, such as in surgical settings, microbiology labs, and pharmaceutical manufacturing.
Making the Right Choice for Your Goal
The decision to boil or autoclave is a decision between sanitization and true sterilization.
- If your primary focus is complete sterilization: You must use an autoclave. It is the only reliable method for destroying bacterial endospores and ensuring the absolute absence of microbial life.
- If your primary focus is sanitization or disinfection: Boiling water is often a sufficient, accessible, and effective method for reducing harmful microorganisms in non-critical applications.
Ultimately, choosing the correct method requires understanding that sterilization isn't just about heat, but about achieving a specific temperature lethal to the most resistant organisms.
Summary Table:
| Method | Max Temperature | Key Capability | Primary Use |
|---|---|---|---|
| Boiling Water | 100°C (212°F) | Disinfection/Sanitization | Household applications, safe water |
| Autoclaving | 121°C (250°F) or higher | True Sterilization | Medical, laboratory, pharmaceutical settings |
Ensure absolute sterility in your laboratory with KINTEK's autoclaves.
Boiling water is insufficient for destroying resilient bacterial spores, a critical requirement in lab and medical environments. KINTEK's reliable autoclaves use pressurized steam to reach the necessary temperatures (121°C/250°F or higher) for guaranteed sterilization, protecting your research integrity and compliance.
Our lab equipment is designed for precision and durability, serving the exacting needs of laboratories like yours. Contact us today to find the perfect sterilization solution for your specific requirements.
Visual Guide
Related Products
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Desktop Fast Laboratory Autoclave Sterilizer 20L 24L for Lab Use
- Laboratory Sterilizer Lab Autoclave Pulsating Vacuum Desktop Steam Sterilizer
- Laboratory High Pressure Steam Sterilizer Vertical Autoclave for Lab Department
- Portable Digital Display Automatic Laboratory Sterilizer Lab Autoclave for Sterilization Pressure
People Also Ask
- What is the minimum temperature and time for autoclave? Achieve Guaranteed Sterilization Every Time
- Why are stainless steel autoclaves key to PCL-TPE preparation? Mastering High-Vacuum Polycondensation
- Why is 121°C used in autoclave? The Science Behind Sterilizing Against Tough Spores
- What core environmental conditions does a supercritical water autoclave provide? Simulating SCWR alloy performance.
- When loading the instruments into the autoclave should you label it? Ensure Safe, Traceable Sterilization Every Time
- What is the 121 cycle of autoclave? A Guide to Guaranteed Sterilization
- Where are autoclaves used? Ensuring Sterility from Hospitals to High-Tech Labs
- How long does autoclaving take? Mastering Sterilization Times for Your Lab



















