Principles of Molecular Distillation
Basic Concept and Operational Conditions
Molecular distillation is a specialized technique that operates under extreme conditions to achieve efficient separation of liquid mixtures. Unlike traditional distillation methods, which often rely on boiling points and atmospheric pressure, molecular distillation operates under high vacuum and low temperatures. This unique operational environment is crucial for several reasons.
First, the high vacuum significantly reduces the atmospheric pressure within the distillation system. At such low pressures, the mean free path of molecules increases, allowing them to travel longer distances without colliding with other molecules. This reduces the likelihood of interactions that could lead to overheating and decomposition, which are common issues in conventional distillation processes.
Second, the low temperatures maintained during molecular distillation help to minimize thermal degradation of the substances being processed. By keeping the temperatures well below the boiling points of the components, the technique ensures that the delicate molecular structures remain intact, preserving the integrity and quality of the final product. This is particularly important for heat-sensitive materials that would otherwise degrade or decompose if subjected to higher temperatures.
In summary, the high vacuum and low-temperature conditions of molecular distillation are designed to prevent the overheating and decomposition that can occur in traditional methods. This makes it an ideal choice for separating and purifying substances that are sensitive to heat and require careful handling to maintain their chemical and physical properties.
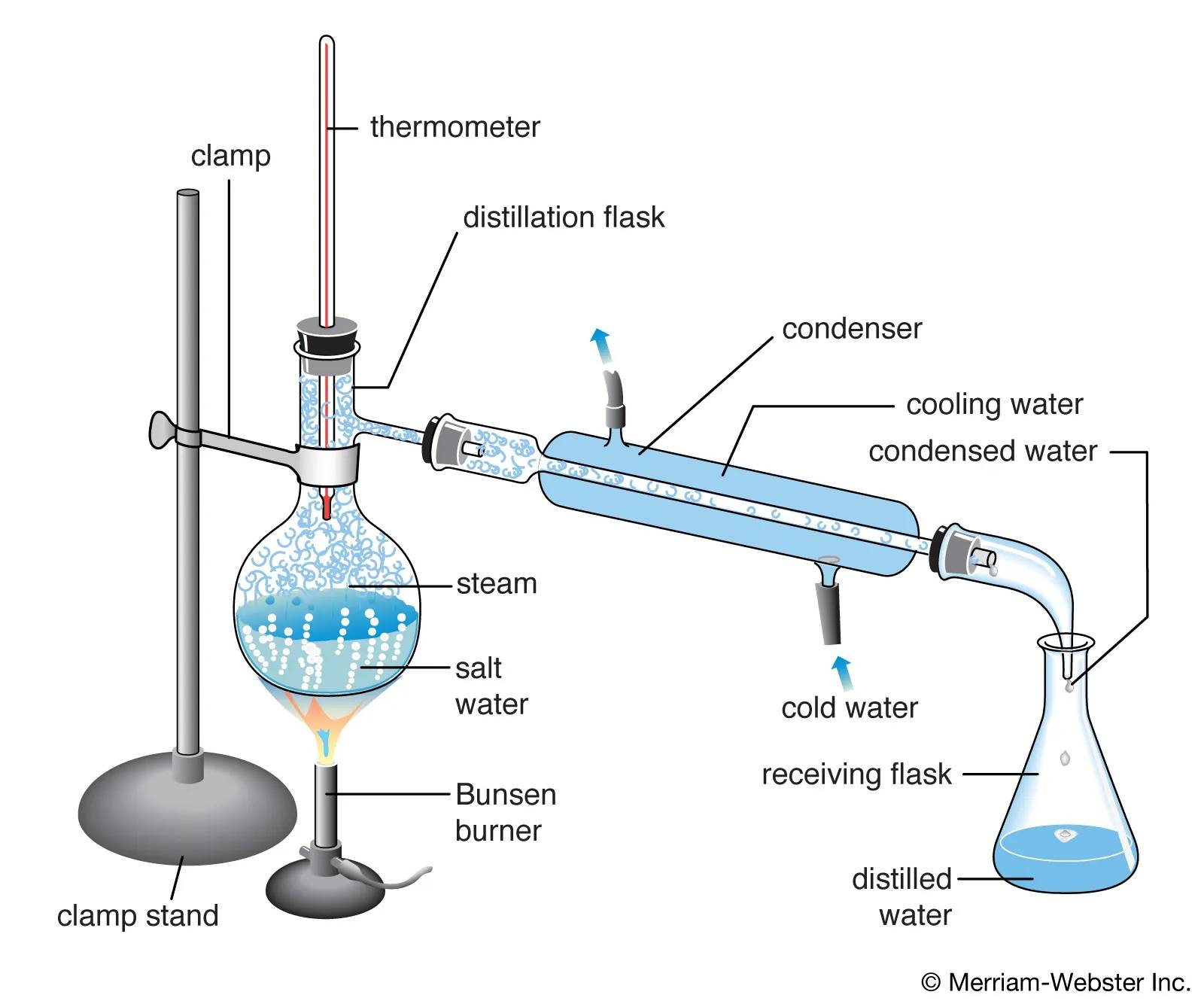
Separation Mechanism
Molecular distillation's separation mechanism is fundamentally rooted in the differences in molecular weights and the forces of interaction between molecules. Under high vacuum conditions, molecules travel in straight lines without frequent collisions, which allows for a more efficient separation process.
Lighter molecules, due to their lower molecular weight, have higher velocities and thus reach the condenser more quickly. Conversely, heavier molecules, with their greater mass, move more slowly and are less likely to escape the distillation kettle. This differential in movement is further accentuated by the intermolecular forces, which can either attract or repel molecules, influencing their trajectories and ultimate destination.
To illustrate, consider a table comparing the behavior of different molecular weights under distillation conditions:
| Molecular Weight | Velocity | Interaction Forces | Destination |
|---|---|---|---|
| Low (Lighter) | High | Weak | Condenser |
| High (Heavier) | Low | Strong | Kettle |
This dynamic interplay ensures that the lighter components are preferentially separated from the heavier ones, achieving a high degree of purification.
Process of Molecular Distillation
Feeding and Diffusion
The liquid mixture, carefully fed into the distillation kettle, undergoes a dynamic process of diffusion and collision due to the thermal energy imparted by the system. Within the kettle, the molecules of the various components in the mixture are subjected to the forces of thermal movement, which cause them to move and interact with each other. This thermal agitation facilitates the diffusion of molecules across the mixture, promoting the mixing and interaction of different components.
As the molecules diffuse, they also collide with one another, exchanging energy and momentum in the process. These collisions are crucial for the separation mechanism of molecular distillation, as they help to distribute the thermal energy evenly among the molecules, enabling the lighter components to gain sufficient energy to escape the mixture more readily than the heavier ones. The continuous diffusion and collision process within the distillation kettle are essential for the efficient operation of molecular distillation, ensuring that the separation of components is based on their molecular properties and interaction forces.
Heating and Condensation
During the molecular distillation process, the liquid mixture is subjected to controlled heating, a critical step that facilitates the separation of components based on their molecular weights and interaction forces. As the mixture is heated, the components gain thermal energy, enabling them to overcome the intermolecular forces that bind them together. This energy allows the molecules to escape from the liquid phase into the vapor phase.
Notably, the lighter molecules, which possess lower boiling points, gain energy more readily and thus escape more easily compared to their heavier counterparts. This differential in escaping rates is a fundamental aspect of molecular distillation, as it allows for the selective separation of components. Once in the vapor phase, these lighter molecules migrate towards the condenser, where they are captured and transformed back into the liquid phase.
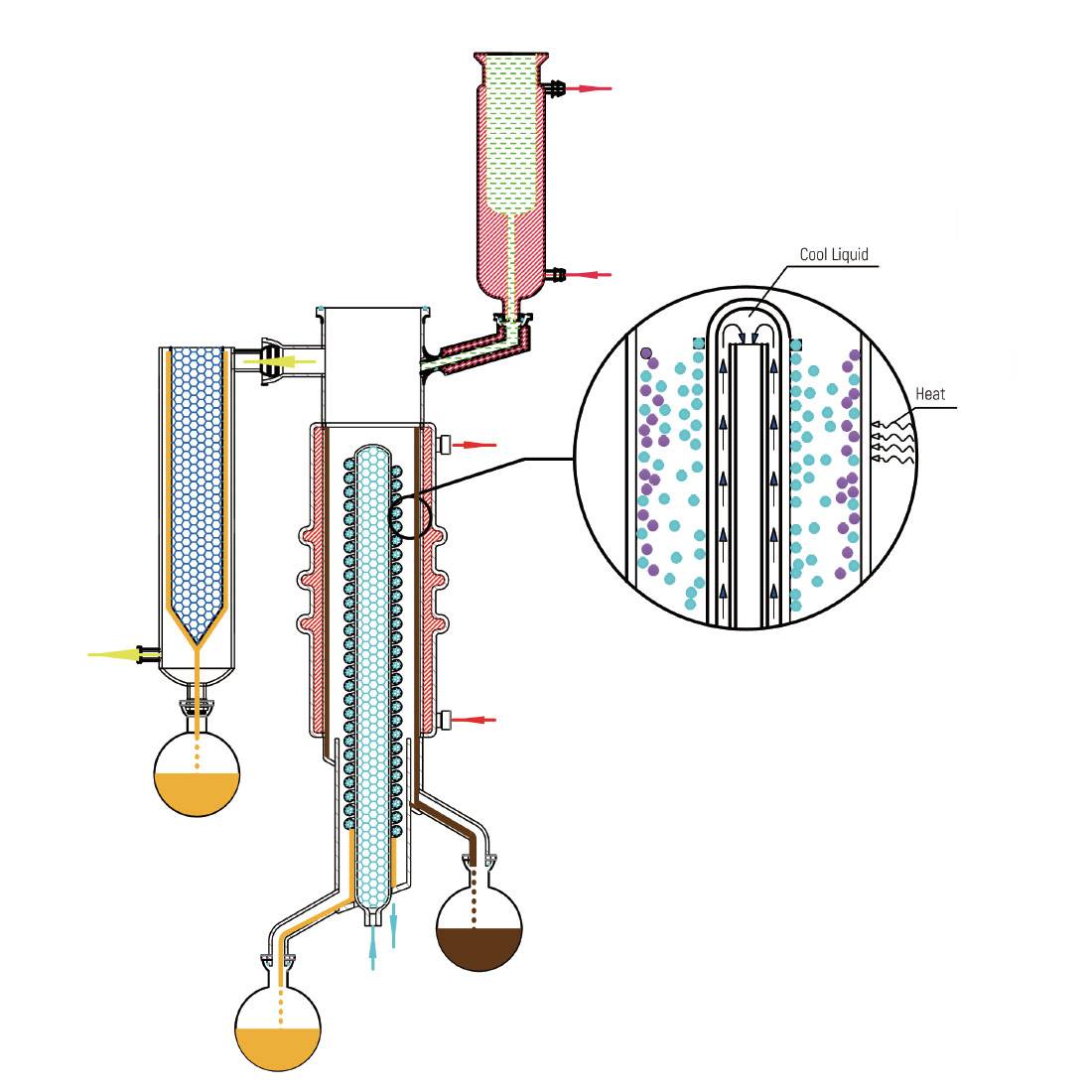
The condenser plays a pivotal role in this process, efficiently capturing the escaping molecules and ensuring that they do not re-enter the distillation system. By maintaining a high vacuum and low temperature environment, molecular distillation minimizes the risk of overheating and decomposition, which are common challenges in traditional distillation methods. This controlled environment not only enhances the efficiency of the separation process but also preserves the integrity and quality of the separated components.
Multi-Stage Distillation
Multi-stage distillation represents a sophisticated approach to enhancing the separation of components within a liquid mixture. By incorporating multiple intermediate condensers, this technique allows for the processing of different fractions at varying temperatures and pressures, thereby optimizing the separation process. This method is particularly advantageous in industries such as chemical, pharmaceutical, and food processing, where the purity of the end product is paramount.
In a typical multi-stage distillation setup, the crude mixture undergoes sequential stages of evaporation and condensation. Each stage is designed to target specific components of the mixture, ensuring that they are separated with precision. For instance, in the distillation of cannabis oil, the terpene fraction can be removed in the initial stage, followed by successive stages that handle the harder and lighter cuts within the same system. This continuous process not only streamlines the operation but also minimizes the risk of contamination and degradation of delicate cannabinoids, which are sensitive to oxygen and light.
The benefits of multi-stage distillation extend beyond operational efficiency. It significantly reduces the exposure of sensitive components to harmful elements, thereby preserving the quality and integrity of the final product. Moreover, the use of multiple stages ensures a more thorough separation, as each stage can be fine-tuned to address the unique properties of different components within the mixture. This is particularly evident in processes like short path distillation system and short path molecular distillation, which often require multiple passes to achieve the desired level of separation.
In essence, the more stages involved in the distillation process, the better the separation outcome. Each additional stage provides an opportunity to refine the separation process, ultimately leading to a higher purity product. This makes multi-stage distillation an indispensable tool in the quest for superior product quality and consistency across various industries.
Applications and Advantages
Industry Usage
Molecular distillation finds extensive application across several industrial sectors, notably in the chemical, pharmaceutical, and food industries. This technique is particularly valued for its ability to purify and separate various substances with high efficiency and minimal thermal damage.
In the chemical industry, molecular distillation is employed to refine raw materials and intermediates, ensuring the production of high-purity chemicals. It is also used in the recycling of solvents and the production of specialty chemicals where purity is critical.
Within the pharmaceutical sector, this method is indispensable for the purification of active pharmaceutical ingredients (APIs). It helps in the removal of impurities and residual solvents, thereby enhancing the safety and efficacy of drugs. The low-temperature operation of molecular distillation is particularly beneficial for heat-sensitive compounds, preventing degradation and maintaining their therapeutic properties.
The food industry also benefits significantly from molecular distillation. It is utilized in the production of high-quality oils, fats, and nutritional supplements. For instance, it is employed in the extraction of essential fatty acids and vitamins from fish oils, ensuring that the final products are free from contaminants and possess enhanced nutritional value.
| Industry | Application |
|---|---|
| Chemical | Refining raw materials, recycling solvents, producing specialty chemicals |
| Pharmaceutical | Purifying APIs, removing impurities and solvents, preserving heat-sensitive compounds |
| Food | Extracting high-quality oils, fats, and nutritional supplements, ensuring product purity |
These applications underscore the versatility and effectiveness of molecular distillation in various industrial processes, making it a crucial technology for modern manufacturing.
Enhancing Product Quality
Molecular distillation plays a pivotal role in refining products by meticulously eliminating impurities and volatile substances. This process significantly enhances product purity and stability, making it indispensable in industries such as pharmaceuticals, chemicals, and food processing. The high vacuum and low-temperature conditions under which molecular distillation operates ensure that the thermal decomposition common in traditional distillation methods is avoided. This preservation of molecular integrity is crucial for maintaining the efficacy and safety of the end products.
For instance, in the pharmaceutical sector, the removal of residual solvents and contaminants through molecular distillation is essential for ensuring drug safety and efficacy. Similarly, in the food industry, this technique is employed to purify oils and fats, thereby enhancing their nutritional value and shelf life. The ability to separate components based on their molecular weights and interaction forces allows for a more precise and effective purification process.
Moreover, the application of molecular distillation extends to specialized fields such as the preparation of high-purity monomers and nanomaterials. These materials often require an exceptional level of purity to exhibit their intended properties and functionalities. Molecular distillation's capability to achieve such high levels of purity makes it a preferred method in these advanced applications.
In summary, molecular distillation not only improves the quality of products by removing impurities but also ensures their stability and integrity, making it a cornerstone in various industrial applications.
Specialized Applications
Molecular distillation finds specialized applications in the preparation of high-purity monomers and nanomaterials, where traditional distillation methods often fall short. This technique excels in environments requiring stringent purity standards due to its ability to operate under high vacuum and low temperatures, which minimizes thermal degradation and contamination.
For monomers, molecular distillation ensures the removal of residual solvents, catalysts, and other impurities that can compromise the polymerization process. This results in polymers with superior mechanical properties, better thermal stability, and enhanced optical clarity. In the realm of nanomaterials, the technique is crucial for isolating and purifying nanoparticles, which often require ultra-clean conditions to maintain their unique properties and functionalities.
Moreover, molecular distillation aids in the synthesis of advanced nanomaterials by facilitating the separation of different molecular species based on their weight and interaction forces. This capability is particularly valuable in the production of quantum dots, carbon nanotubes, and other nanostructured materials, where even trace amounts of impurities can significantly alter their electronic, optical, and magnetic properties.
In summary, molecular distillation's specialized applications in high-purity monomers and nanomaterials underscore its indispensable role in advancing material science and technology.
Challenges and Future Prospects

Limitations
Molecular distillation, while highly effective for many types of separations, does face significant challenges when dealing with substances that possess unique properties or exhibit high viscosity. These substances often present difficulties in the separation process due to their inherent characteristics, which can hinder the diffusion and collision mechanisms essential for effective distillation.
For instance, substances with high viscosity tend to flow slowly, which can impede the movement of molecules within the distillation apparatus. This sluggish movement can lead to inefficient diffusion, where molecules do not readily separate based on their molecular weights and intermolecular forces. As a result, the desired separation of lighter components from heavier ones becomes less effective, compromising the overall efficiency of the distillation process.
Moreover, substances with special properties, such as those exhibiting strong intermolecular interactions or being highly reactive, can further complicate the separation process. These interactions can create a more cohesive mixture, making it challenging for the molecules to diffuse and escape the distillation kettle. Additionally, the reactivity of certain substances can lead to unwanted side reactions or decomposition, which not only affects the purity of the final product but also poses safety risks during the distillation operation.
In summary, while molecular distillation is a powerful technique for many separation tasks, its effectiveness is significantly limited when dealing with substances that are highly viscous or possess unique, challenging properties. Addressing these limitations requires ongoing research and technological advancements to optimize the process and expand its applicability to a broader range of substances.
Environmental and Energy Concerns
Addressing pollution and energy consumption issues in the distillation process is crucial for the sustainable application of molecular distillation. The high vacuum and low-temperature conditions required by molecular distillation can lead to significant energy consumption, which, if not managed efficiently, can have detrimental environmental impacts.
To mitigate these concerns, several strategies can be employed:
-
Energy Efficiency Improvements:
- Heat Recovery Systems: Implementing heat exchangers can recycle and reuse the thermal energy generated during the distillation process, thereby reducing overall energy consumption.
- Optimized Operational Parameters: Fine-tuning the vacuum levels and temperature settings can minimize energy usage without compromising the separation efficiency.
-
Pollution Control Measures:
- Emission Reduction Technologies: Installing scrubbers and filters can capture and neutralize volatile organic compounds (VOCs) and other pollutants released during the process.
- Waste Management: Proper disposal and recycling of distillation residues can prevent environmental contamination and promote resource conservation.
-
Sustainable Practices:
- Renewable Energy Integration: Utilizing renewable energy sources such as solar or wind power can offset the carbon footprint associated with energy consumption.
- Green Chemistry Principles: Adopting green chemistry practices in the formulation of feed materials can reduce the generation of hazardous by-products.
By adopting these strategies, the molecular distillation process can be made more environmentally friendly and energy-efficient, aligning with global sustainability goals.
Technological Advancements
Future innovations in molecular distillation are poised to revolutionize the process, focusing on enhancing separation effects, minimizing energy consumption, and fostering sustainable development. These advancements are expected to address some of the current limitations, particularly those related to the separation of substances with unique properties or high viscosity.
One promising area of research involves the integration of advanced materials and smart technologies. For instance, the development of new coatings for distillation equipment could improve heat transfer efficiency and reduce the adhesion of viscous substances, thereby enhancing separation efficiency. Additionally, the use of nanotechnology could lead to the creation of more effective filters and membranes, allowing for the precise separation of components based on their molecular characteristics.
Energy efficiency is another critical focus. Innovations in heat recovery systems and the optimization of operational parameters under high vacuum conditions are being explored to reduce the overall energy footprint of the distillation process. These efforts not only lower operational costs but also contribute to environmental sustainability by reducing greenhouse gas emissions and other pollutants associated with energy production.
Moreover, the adoption of automation and machine learning algorithms in the control systems of molecular distillation units is anticipated to streamline operations and improve process accuracy. These technologies can predict and adjust for variations in feedstock composition and operational conditions, ensuring consistent and high-quality outputs.
In summary, the future of molecular distillation lies in the synergy of advanced materials, smart technologies, and sustainable practices, all aimed at optimizing performance while minimizing environmental impact.
Related Products
- Custom PTFE Teflon Parts Manufacturer for Sampling Filters
- Laboratory Wet Three-Dimensional Vibratory Sieve Shaker Machine
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Filter Testing Machine FPV for Dispersion Properties of Polymers and Pigments
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
Related Articles
- PTFE Shovel: A Comprehensive Guide to Its Uses, Advantages, and Applications
- Innovative Application of PTFE in Mechanical Seals
- Applications of Dry Cold Traps in Various Processes
- Versatile Applications of PTFE Cleaning Racks: Enhancing Laboratory Efficiency and Precision
- PTFE Cleaning Racks: The Ultimate Guide to Cleaning and Drying Labware





