Introduction to Molecular Distillation
Definition and Applications
Molecular Distillation (MD), also known as Short Path Distillation, is a sophisticated separation and purification technology that operates under high vacuum conditions. This method leverages the average free path of molecular movement to achieve separation, distinguishing it from traditional distillation techniques that rely on differences in boiling points. MD is renowned for its high efficiency and precision, making it indispensable across multiple industries.
In the petroleum sector, MD is employed for the separation and purification of heavy oils, ensuring the production of high-purity petrochemical intermediates with minimal environmental impact. Similarly, in the chemical industry, it aids in the purification of complex mixtures, enhancing the quality and yield of products.
The food industry benefits significantly from MD, particularly in the separation of essential oil components and mixed oils. This technology is crucial for the production of high-purity products such as monoglycerides and polyunsaturated fatty acids, which are integral to various food applications.
In the pharmaceutical industry, MD plays a pivotal role in the production of key compounds like Vitamin E, squalene, and long-chain fatty alkanols. Its ability to operate at low temperatures ensures the preservation of sensitive compounds, while its high separation degrees guarantee the purity and efficacy of the final products.
Moreover, in the fine chemical and new material industries, MD is applied in the production of essential oils and high-performance fiber raw materials, respectively. In these contexts, MD not only ensures high separation efficiency but also maintains the integrity and quality of the extracts, thereby contributing to the development of advanced materials.
Principle of Molecular Distillation
Molecular distillation operates under high vacuum conditions, leveraging the average free path of molecular movement to achieve separation. This process differs significantly from general distillation, which relies on differences in boiling points to separate components. In molecular distillation, the vacuum environment allows the mean free path of vapor molecules to exceed the distance between the evaporation surface and the condensation surface. This setup enables the separation of liquid mixtures by exploiting the varying evaporation rates of each component.
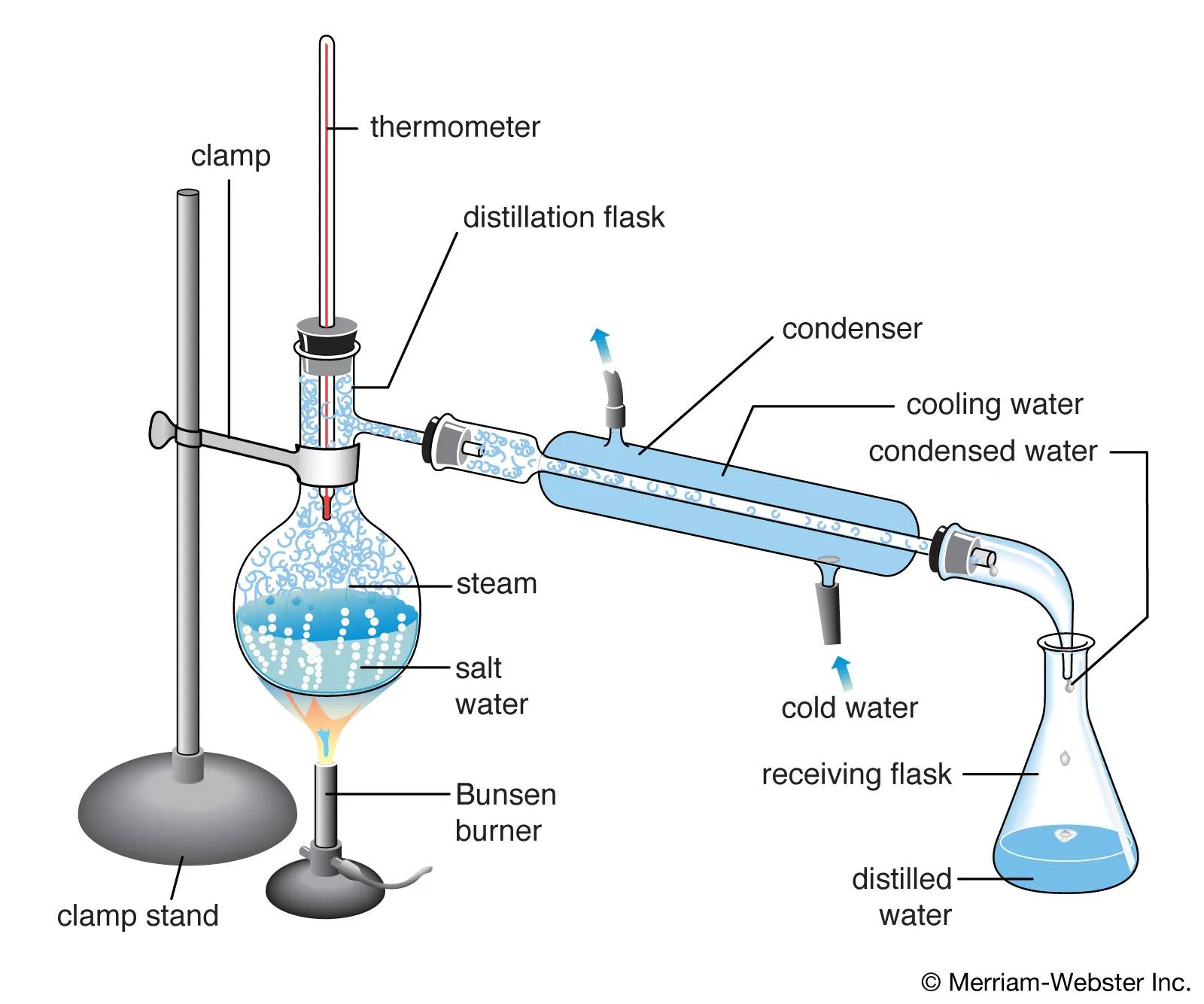
The principle behind molecular distillation can be further understood through the concept of molecular mean free path. According to thermodynamic principles, the mean free path ( L ) of molecules is given by the formula:
[ L = \frac{0.707K \cdot T}{\pi d^2 P} ]
where:
- ( L ) is the mean free path,
- ( K ) is the Boltzmann constant,
- ( T ) is the temperature,
- ( d ) is the effective molecular diameter,
- ( P ) is the pressure in the molecular space.
This formula illustrates that different molecules, due to their distinct effective diameters, have varying mean free paths. Consequently, the distance a molecule can travel without colliding with another molecule differs. By strategically positioning a condensing surface closer to the liquid surface than the mean free path of lighter molecules, molecular distillation can selectively condense lighter molecules while allowing heavier molecules to return to the liquid surface. This mechanism effectively separates the mixture, achieving high precision and efficiency in the process.
Technical Details and Equipment
Main Equipment Components
The molecular distillation system is a sophisticated assembly of key components designed to facilitate high-efficiency separation and purification processes. At its core, the system comprises a molecular distiller, which is the central unit responsible for the actual distillation process. This distiller operates under high vacuum conditions, ensuring that the separation occurs at molecular levels, thereby achieving a high degree of purity.
Complementing the molecular distiller is the degassing system, which is crucial for removing any dissolved gases from the feedstock before it enters the distillation process. This step is essential to prevent bubble formation, which could disrupt the distillation process and reduce the efficiency of the system.
The feeding system is another critical component, designed to introduce the feedstock into the molecular distiller at a controlled rate. This system ensures that the distillation process is consistent and that the feedstock is evenly distributed across the distiller, maximizing the efficiency of the separation process.
To facilitate the distillation, the system includes a heating system that provides the necessary thermal energy to vaporize the feedstock. This heating system is typically designed to operate at precise temperatures, ensuring that the distillation occurs at the optimal conditions for the specific feedstock being processed.
The condensing system is responsible for cooling the vaporized molecules and converting them back into a liquid state. This system is crucial for collecting the purified fractions and ensuring that they are of the highest possible quality. The condensing system often includes multiple stages to achieve different degrees of separation, depending on the specific requirements of the process.
The fraction collection system is designed to collect and store the various fractions produced during the distillation process. This system ensures that each fraction is isolated and stored separately, allowing for further processing or use as needed.
Maintaining the high vacuum conditions required for molecular distillation is the responsibility of the vacuum system. This system includes pumps and other equipment designed to remove air and other gases from the distillation environment, ensuring that the process occurs under the optimal conditions for molecular separation.
Finally, the control system acts as the brain of the molecular distillation system, monitoring and regulating all the components to ensure that the process runs smoothly and efficiently. This system includes sensors, controllers, and other devices that provide real-time data and allow for adjustments to be made as needed to maintain optimal performance.

Scraped Membrane Molecular Distillers
Scraped film molecular distillers are a cornerstone in the field of molecular distillation, characterized by their robust design and efficient operation. These distillers typically include a cylindrical vessel equipped with a jacket for temperature control, a sophisticated rotor system, and an internal condenser. The cylindrical shape facilitates uniform heat distribution, ensuring optimal thermal efficiency during the distillation process.
The rotor system, often comprising multiple blades or scrapers, plays a crucial role in maintaining the integrity of the distilling material. By continuously scraping the inner surface of the cylinder, it prevents the buildup of deposits that could otherwise hinder the process. This mechanical action not only enhances heat transfer but also ensures a consistent surface area for evaporation and condensation, thereby maintaining high separation efficiency.
The internal condenser, strategically positioned within the distiller, allows for immediate condensation of the evaporated molecules, minimizing the distance they need to travel. This short path distillation principle is key to the high precision and efficiency of molecular distillation. The combination of these elements—the cylindrical vessel, rotor system, and internal condenser—enables scraped film molecular distillers to operate under high vacuum conditions, ensuring low separation temperatures and high purity of the distillate.
Characteristics and Advantages
Key Features
Molecular distillation stands out due to its unique operational characteristics that set it apart from traditional distillation methods. One of its most significant features is the low separation temperatures it employs. This is particularly beneficial for heat-sensitive materials, as it minimizes thermal degradation and preserves the integrity of the compounds being processed.
Operating under high vacuum conditions is another hallmark of molecular distillation. This high vacuum environment allows for the separation of molecules based on their mean free path, rather than relying on differences in boiling points. This mechanism ensures that the process is both efficient and precise.
Short heating times are also a key advantage. The rapid heating and cooling cycles inherent in molecular distillation reduce the overall process time, thereby enhancing productivity and reducing energy consumption. This efficiency is further bolstered by the high separation degrees achieved, which result in highly purified end products.
Unlike conventional distillation, molecular distillation is irreversible. This means that once the separation process is complete, the components cannot revert to their original mixed state, ensuring the purity and stability of the final products. Additionally, the absence of boiling or bubbling phenomena during the process eliminates the risk of product degradation due to excessive heat and pressure.
These features collectively make molecular distillation an ideal choice for industries requiring high-precision separation and purification of complex mixtures, particularly those involving heat-sensitive and high-value compounds.
Environmental and Efficiency Benefits
Molecular distillation stands out as a green technology, significantly reducing environmental impact while enhancing operational efficiency. Unlike traditional distillation methods, molecular distillation operates under high vacuum conditions, which minimizes energy consumption and heat-induced degradation of sensitive compounds. This process ensures that volatile organic compounds (VOCs) are contained within the system, preventing their release into the atmosphere and thereby contributing to cleaner air quality.
Moreover, the high efficiency of molecular distillation translates into substantial cost savings and increased productivity. By utilizing short heating times and low separation temperatures, this technology can handle complex mixtures with precision, yielding high-purity products without the need for extensive additional treatments. This not only reduces waste but also streamlines the production process, making it more sustainable and economically viable.
In summary, molecular distillation not only meets modern environmental standards but also offers a competitive edge through its superior efficiency and effectiveness in complex separation tasks.
Applications Across Industries
Pharmaceutical Industry
Molecular distillation plays a crucial role in the pharmaceutical industry, particularly in the production of high-value compounds such as Vitamin E, squalene, long-chain fatty alkanols, and patchouli essential oil. This advanced separation technology offers several distinct advantages, including low operating temperatures and high separation degrees, which are essential for maintaining the integrity and efficacy of these sensitive compounds.
One of the primary benefits of using molecular distillation in pharmaceutical manufacturing is its ability to operate at low temperatures. This is particularly important for heat-sensitive materials, as it prevents thermal degradation and preserves the bioactive properties of the compounds. For instance, Vitamin E, which is prone to oxidation at high temperatures, can be efficiently purified using molecular distillation without compromising its stability and potency.

Moreover, the high separation degrees achieved through molecular distillation ensure that the final products are of high purity and quality. This is especially critical in the pharmaceutical sector, where even trace impurities can significantly impact the efficacy and safety of drugs. For example, the purification of squalene, a compound used in various pharmaceutical formulations, benefits from the high separation efficiency of molecular distillation, resulting in a product that meets stringent purity standards.
In addition to Vitamin E and squalene, molecular distillation is also employed in the production of long-chain fatty alkanols and patchouli essential oil. These compounds are used in a variety of pharmaceutical applications, including as active ingredients in topical creams and ointments. The ability of molecular distillation to efficiently separate and purify these compounds ensures that the final products are consistent in quality and efficacy.
Overall, molecular distillation stands out as a superior purification method in the pharmaceutical industry, offering a combination of low operating temperatures and high separation degrees that are essential for the production of high-quality, bioactive compounds.
Petrochemical Industry
In the petrochemical industry, molecular distillation plays a pivotal role in the separation and purification of heavy oils and petrochemical intermediates. This process is particularly crucial for refining crude oil into high-value products such as lubricants, waxes, and specialty chemicals. By operating under high vacuum conditions, molecular distillation ensures that these products are obtained with exceptionally high purity, significantly reducing the presence of contaminants and impurities.
One of the standout benefits of using molecular distillation in this sector is its ability to minimize environmental impact. Traditional distillation methods often result in the release of volatile organic compounds (VOCs) and other pollutants into the atmosphere. In contrast, molecular distillation operates at lower temperatures and under vacuum, which helps to contain and reduce these emissions, making it a more sustainable and environmentally friendly option.
Moreover, the efficiency of molecular distillation in the petrochemical industry extends beyond just environmental benefits. The technology allows for the recovery of valuable by-products that might otherwise be lost in conventional distillation processes. This not only enhances the overall yield but also contributes to cost savings by reducing waste and increasing the utilization rate of raw materials.
In summary, molecular distillation is a transformative technology in the petrochemical industry, offering a combination of high-purity product outputs, reduced environmental footprint, and operational efficiencies that are unmatched by traditional methods.
Food Industry
Molecular distillation plays a pivotal role in the food industry by refining essential oils and mixed oils, thereby elevating the purity of various products. This advanced separation technique is particularly beneficial for isolating monoglycerides and polyunsaturated fatty acids, which are critical components in many food formulations.
Key Applications in the Food Industry
- Essential Oil Separation: Molecular distillation effectively separates the components of essential oils, ensuring that the final product retains its aromatic and therapeutic qualities without contamination from unwanted compounds.
- Mixed Oil Purification: In the processing of mixed oils, molecular distillation helps to purify the oil by removing impurities and enhancing the concentration of beneficial fatty acids.
- Monoglycerides and Polyunsaturated Fatty Acids: The technique is instrumental in producing high-purity monoglycerides, which are used as emulsifiers, and polyunsaturated fatty acids, which are important for human health.

Benefits of Molecular Distillation in Food Processing
- High Purity Products: The process ensures that the end products meet stringent purity standards, which is crucial for maintaining the quality and safety of food products.
- Minimal Thermal Damage: Operating under high vacuum conditions, molecular distillation minimizes thermal damage to heat-sensitive components, preserving the nutritional value and sensory properties of the food.
- Efficient Separation: The technique offers a high degree of separation efficiency, reducing the need for multiple purification steps and thus lowering production costs.
Molecular distillation not only enhances the quality of food products but also contributes to the development of healthier and more sustainable food options, making it an indispensable technology in modern food processing.
Fine Chemical Industry
In the fine chemical industry, molecular distillation stands out as a pivotal technology for the production of high-quality essential oils, such as oregano, rosemary, and pepper. This advanced distillation technique operates under high vacuum conditions, which significantly reduces the separation temperature compared to traditional methods. As a result, it preserves the delicate aromatic compounds and natural constituents of these oils, ensuring that their therapeutic and sensory properties remain intact.
Molecular distillation's unique ability to handle high-viscosity materials without causing thermal degradation is particularly advantageous in this sector. For instance, the process effectively separates and purifies the complex mixtures found in essential oils, yielding products with superior purity and potency. This not only enhances the overall quality of the final products but also ensures consistent yields, making it a preferred method for manufacturers seeking to meet stringent industry standards.
Moreover, the application of molecular distillation in the fine chemical industry extends beyond essential oils. It is also utilized in the production of various other high-value chemical compounds, where maintaining the integrity and purity of the end product is critical. The technology's ability to operate at lower temperatures and its high separation efficiency make it an indispensable tool in this field, contributing to the development of innovative and high-performance fine chemicals.
New Material Industry
In the realm of high-performance fiber raw materials, molecular distillation emerges as a pivotal technology, ensuring not only high separation efficiency but also the preservation of the integrity and quality of the extracts. This advanced separation technique operates under high vacuum conditions, leveraging the average free range of molecular movement to achieve precise separations. Unlike conventional distillation methods that rely on boiling points, molecular distillation excels in its ability to handle temperature-sensitive materials without compromising their structural integrity.
The application of molecular distillation in the new material industry is particularly significant for the production of high-performance fibers, where the purity and consistency of raw materials are critical. For instance, in the manufacturing of advanced composites and specialty fibers, the ability to maintain the molecular structure of the raw materials is essential. Molecular distillation facilitates this by providing a clean, efficient, and environmentally friendly process that minimizes thermal degradation and oxidation, thereby enhancing the overall quality and performance of the final products.
Moreover, the technology's inherent advantages, such as short heating times and high separation degrees, make it an ideal choice for industries requiring high-purity extracts. This not only reduces the risk of contamination but also ensures that the end products meet stringent quality standards. The integration of molecular distillation in the new material industry underscores its versatility and effectiveness in delivering superior raw materials for advanced applications.
Related Products
- Wall Mounted Water Distillation Unit
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Custom PTFE Teflon Parts Manufacturer for Sampling Filters
- Custom PTFE Teflon Parts Manufacturer for PTFE Stirring Bar Recovery Rod
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
Related Articles
- How Rotary Evaporator is Different from Conventional Distillation
- What Is Multiple Effect Evaporator?
- Understanding the Science Behind Short Path Distillation
- The Importance of Water Distillation in the Laboratory: Ensuring Purity and Quality for Accurate Results
- How Rotary Evaporators are Revolutionizing Distillation








