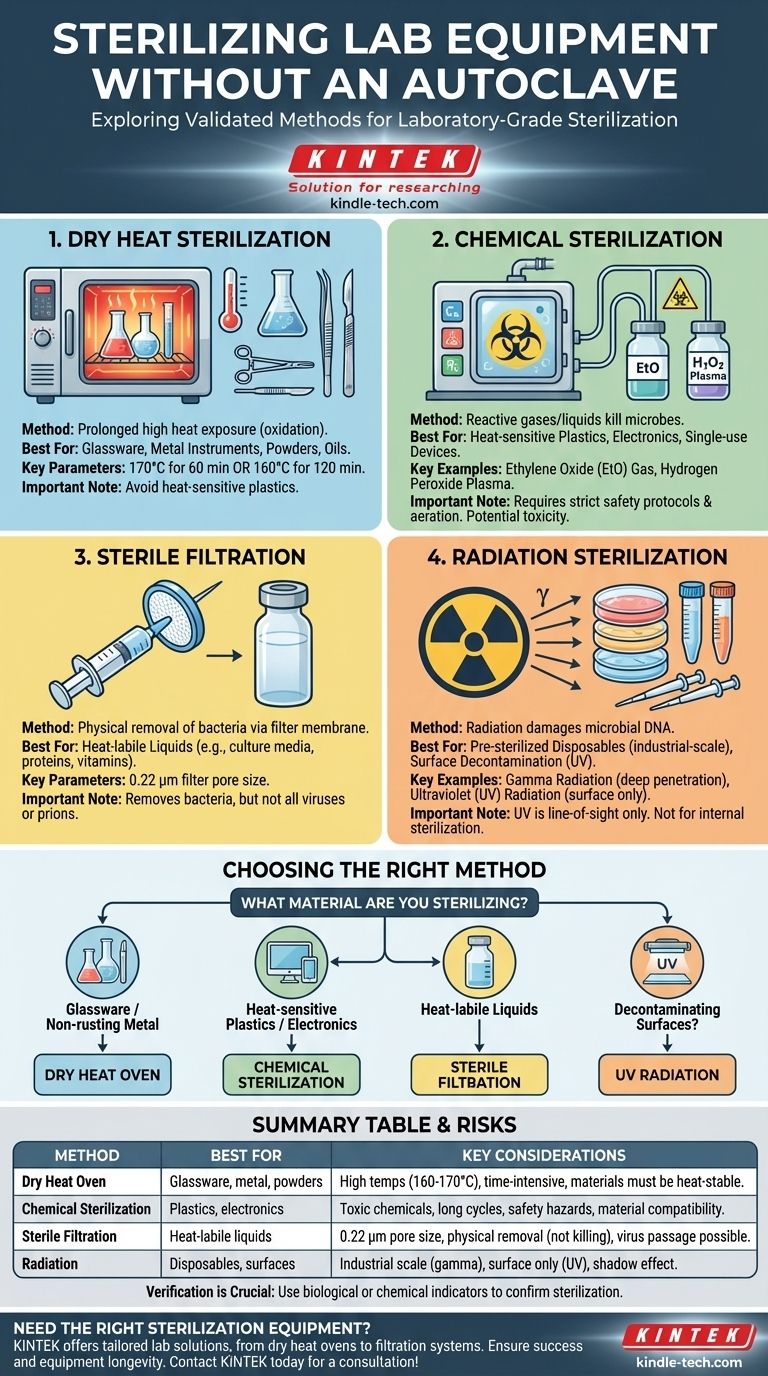
When an autoclave is unavailable or unsuitable, you can still achieve laboratory-grade sterilization using several validated methods. The most common alternatives include dry heat sterilization in an oven, chemical sterilization using gases or liquids, radiation, and sterile filtration for liquids. The choice of method is dictated entirely by the material you need to sterilize, as some items like heat-sensitive plastics cannot withstand the high-pressure steam of an autoclave.
The core principle of sterilization is not tied to a single machine, but to the successful elimination of all microbial life. The best alternative to an autoclave is the method that achieves this goal without damaging your equipment or samples, requiring a careful match between the material and the method.

Why Choose an Alternative to Autoclaving?
While autoclaving (steam sterilization) is the gold standard for many applications, it is not a universal solution. Understanding its limitations is key to selecting the appropriate alternative.
Material Incompatibility
The combination of high heat (typically 121°C or higher) and pressurized steam can damage certain materials. This includes heat-labile plastics that may melt, sensitive electronics, and sharp metal instruments that can be dulled by repeated steam exposure.
Logistical or Equipment Constraints
In some settings, an autoclave may simply be unavailable, out of service for maintenance, or too small for the equipment you need to process. A reliable alternative is essential for continuing work without interruption.
Primary Sterilization Methods Without an Autoclave
Each method operates on a different principle and is suited for specific types of equipment and materials.
Dry Heat Sterilization
This method uses a laboratory oven to kill microbes with high heat over a prolonged period. It works by denaturing proteins through oxidation, which requires higher temperatures and longer exposure times than steam heat.
It is ideal for glassware (like flasks and beakers), metal instruments, powders, and oils. Typical cycles are 170°C (340°F) for 60 minutes or 160°C (320°F) for 120 minutes.
Chemical Sterilization (Cold Sterilization)
This approach uses reactive chemicals, often in gaseous form, to sterilize items that cannot withstand heat. It is a common choice for single-use medical devices and sensitive lab equipment.
Key examples include Ethylene Oxide (EtO) gas, which is highly effective but toxic and requires a long aeration period, and hydrogen peroxide plasma, which is faster and safer but has material compatibility limitations. Liquid sterilants like glutaraldehyde are also used for soaking instruments.
Sterile Filtration
Filtration does not kill microbes; it physically removes them. This method is used exclusively for liquids, such as culture media, protein solutions, or vitamin supplements that would be destroyed by heat.
The liquid is passed through a filter membrane with a pore size small enough to trap bacteria, typically 0.22 micrometers (µm). This renders the filtrate sterile, but note that very small viruses or prions may still pass through.
Radiation Sterilization
Radiation damages the DNA of microorganisms, preventing them from replicating. This is a highly effective, industrial-scale method used to pre-sterilize many disposable lab products.
Gamma radiation is the most common form, used for items like plastic petri dishes, centrifuge tubes, and pipette tips. Ultraviolet (UV) radiation is a less penetrating form used for surface decontamination inside biosafety cabinets, but it is not effective for sterilizing entire objects.
Understanding the Trade-offs and Risks
No method is without its downsides. Choosing an alternative to autoclaving requires a clear understanding of the potential risks and limitations.
Risk of Material Damage
Dry heat can melt plastics and warp other materials that might be stable in an autoclave. Conversely, chemical sterilants like ethylene oxide can be absorbed by some plastics, requiring lengthy aeration, while hydrogen peroxide can corrode certain metals.
Chemical and Safety Hazards
Chemical sterilants are often toxic, carcinogenic, or highly reactive. Using methods like Ethylene Oxide requires specialized, vented chambers and strict safety protocols to protect personnel from exposure.
Incomplete Sterilization
If not performed correctly, alternative methods can fail. Dry heat requires precise time and temperature control, as air is a less efficient conductor of heat than steam. UV radiation is only effective on surfaces with direct line-of-sight exposure; any "shadowed" areas will not be decontaminated.
The Need for Verification
Just as with an autoclave, you must verify that your chosen method was successful. This involves using biological indicators (spores of a highly resistant bacterium) or chemical indicators that change color to confirm that sterilizing conditions were met.
Choosing the Right Method for Your Goal
Select your sterilization process based on the material you are handling and the resources you have available.
- If you are sterilizing glassware or non-rusting metal instruments: Use a dry heat oven, as it is effective and straightforward for heat-stable, non-aqueous items.
- If you are sterilizing heat-sensitive plastics or complex electronics: Chemical sterilization is the most appropriate method, though it demands specialized equipment and strict safety protocols.
- If you are sterilizing liquids containing heat-labile components (e.g., vitamins, antibiotics): Use sterile filtration with a 0.22 µm filter to remove bacteria without destroying the sensitive compounds.
- If you are decontaminating a work surface or the air in a contained space: Use UV radiation, but understand its limitations for sterilizing solid, three-dimensional objects.
By carefully matching the sterilization method to the material, you ensure the integrity of both your equipment and your experimental results.
Summary Table:
| Method | Best For | Key Considerations |
|---|---|---|
| Dry Heat Oven | Glassware, metal instruments, powders, oils | Requires 160-170°C for 1-2 hours; avoid heat-sensitive plastics |
| Chemical Sterilization | Heat-sensitive plastics, electronics, single-use devices | Uses EtO gas or H2O2 plasma; requires safety protocols and aeration |
| Sterile Filtration | Heat-labile liquids (media, proteins, vitamins) | Uses 0.22 µm filter; removes bacteria but not all viruses |
| Radiation | Pre-sterilized disposables (petri dishes, pipette tips) | Industrial-scale (gamma) or surface decontamination (UV) |
Need the Right Sterilization Equipment for Your Lab?
Choosing the correct method is critical for your experiments' success and equipment longevity. KINTEK specializes in lab equipment and consumables, offering solutions tailored to your specific sterilization needs—from durable dry heat ovens to reliable filtration systems. Let our experts help you select the ideal setup to maintain a sterile environment without compromising your materials.
Contact KINTEK today for a personalized consultation!
Visual Guide
Related Products
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Sterilizer Lab Autoclave Pulsating Vacuum Desktop Steam Sterilizer
- Laboratory Sterilizer Lab Autoclave Herbal Powder Sterilization Machine for Plant
- Portable Digital Display Automatic Laboratory Sterilizer Lab Autoclave for Sterilization Pressure
People Also Ask
- Why is a high-pressure hydrothermal autoclave core for g-C3N4/CeO2? Achieve Powerful Heterojunction Synthesis
- What is the use of autoclave in research? Ensure Sterile Conditions for Valid Scientific Results
- What are the four components of sterilization? Master the Pillars of Effective Sterilization
- How long are autoclaved items sterile? Understanding Event-Related Sterility for Lab Safety
- Which critical experimental conditions does a high-pressure autoclave provide? Optimize Mixed Sulfide Leaching
- Why is an autoclave simulation system necessary for evaluating zirconium cladding? Ensure Nuclear Safety and Longevity
- How long does it take for the autoclave to complete its cycle? From 30 Minutes to Over an Hour
- Why must Ni-Cr alloy and carbon-coated samples be treated in a pressure steam autoclave? Ensure Data Integrity



















