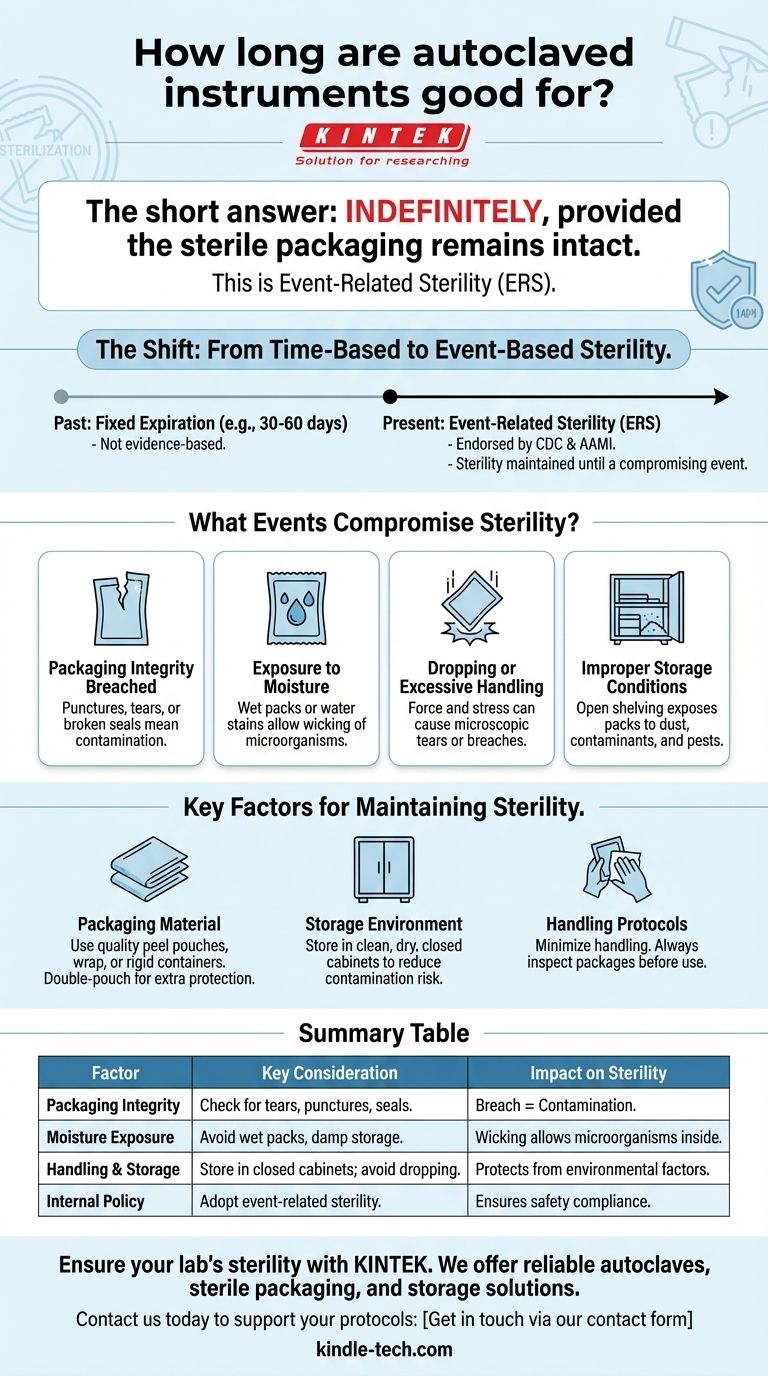
The short answer is indefinitely, provided the sterile packaging remains intact. Modern infection control has moved away from assigning a fixed expiration date to autoclaved items, adopting a more logical standard known as "event-related sterility." This means an item is considered sterile until an "event" occurs that compromises the integrity of its packaging.
The core principle is that the passage of time does not cause a sterile item to become non-sterile. Contamination is caused by physical events: a tear in the packaging, exposure to moisture, or excessive handling. Therefore, your focus should be on proper handling and storage, not on a calendar date.

The Shift from Time-Based to Event-Based Sterility
For decades, healthcare facilities applied time-related expiration dates to sterile packages, such as 30 or 60 days. This practice has been largely replaced because it is not supported by scientific evidence.
Why Fixed Dating is Obsolete
The old model assumed that a package's sterility "expired" after a set period. However, studies showed no correlation between the time an item was stored and the likelihood of it being contaminated, as long as the packaging was not compromised.
This led governing bodies like the CDC and the Association for the Advancement of Medical Instrumentation (AAMI) to endorse event-related sterility (ERS) as the standard. ERS is a more reliable and logical system for ensuring patient safety.
What Events Compromise Sterility?
Event-related sterility requires you to assess the integrity of each package before use. An item must be considered contaminated and in need of reprocessing if any of the following events have occurred.
Packaging Integrity is Breached
This is the most obvious cause of contamination. Look for any punctures, tears, or broken seals. A package that has been crushed or has a damaged seal is no longer sterile.
Exposure to Moisture
Moisture is a primary vehicle for contamination. If a sterile pack becomes wet, it creates a "wicking" effect, allowing microorganisms to travel from the outside of the package to the inside. A package that is wet, has water stains, or was stored in a damp location is compromised.
Dropping or Excessive Handling
When a package is dropped on the floor, the force of the impact can cause microscopic tears or force airborne particles through the material. Similarly, excessive handling or shuffling of packs can stress the seals and surfaces, potentially leading to a breach.
Improper Storage Conditions
The storage environment is critical. Open shelving exposes packs to dust, airborne contaminants, and potential damage. Items should be stored in clean, dry, closed cabinets or covered containers to protect them from environmental factors, including pests and vermin.
Understanding the Key Factors for Maintaining Sterility
While time itself is not the factor, your choices in packaging and storage directly influence how long a package can realistically withstand potential contaminating events.
The Role of Packaging Material
The quality of your packaging is your first line of defense. Sterilization peel pouches, wrap, and rigid containers are all designed to maintain a barrier against microorganisms.
Double-pouching or using multiple layers of wrap can provide additional protection against handling and environmental challenges, but it does not make a package immune to mishandling or moisture.
The Importance of the Storage Environment
A controlled environment is non-negotiable. Sterile storage areas must be clean, dry, and have controlled temperature and humidity. Storing items in closed cabinets drastically reduces the risk of contamination compared to open shelving.
The Human Factor: Handling Protocols
Your team's handling procedures are just as important as the physical environment. Protocols should mandate minimal handling of sterile items. Always inspect every package carefully before opening it at the point of use.
Making the Right Choice for Your Practice
Implementing an event-related sterility policy requires a shift in mindset from checking dates to inspecting packages.
- If your primary focus is maximum patient safety and compliance: Your policy must clearly define what constitutes a contaminating "event" and mandate that all staff inspect every package's integrity before use.
- If your primary focus is practical implementation in a clinic or lab: Prioritize investing in proper storage, such as closed cabinets or dust covers for shelving, and train staff on gentle handling protocols.
- If your primary focus is creating an internal policy: Document your adherence to event-related sterility, detailing the inspection criteria (checking for tears, moisture, and seal integrity) and the procedure for reprocessing a compromised item.
Ultimately, sterility is an active process of protection and inspection, not a passive state with an expiration date.
Summary Table:
| Factor | Key Consideration | Impact on Sterility |
|---|---|---|
| Packaging Integrity | Check for tears, punctures, or broken seals. | A breach means immediate contamination. |
| Moisture Exposure | Avoid wet packs, water stains, or damp storage. | Moisture allows microorganisms to wick inside. |
| Handling & Storage | Store in clean, dry, closed cabinets; avoid dropping. | Protects from dust, damage, and environmental factors. |
| Internal Policy | Adopt event-related sterility, not fixed expiration dates. | Ensures compliance with CDC/AAMI standards for safety. |
Ensure your lab's sterility and compliance with the right equipment. Proper autoclaving is just the first step; maintaining sterility requires quality packaging, correct handling, and suitable storage solutions. KINTEK specializes in lab equipment and consumables, serving laboratory needs with reliable autoclaves, sterile packaging, and storage cabinets designed to protect your instruments.
Contact us today to discuss how we can support your infection control protocols and enhance your lab's safety and efficiency. Get in touch via our contact form!
Visual Guide
Related Products
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Laboratory High Pressure Steam Sterilizer Vertical Autoclave for Lab Department
- Laboratory Sterilizer Lab Autoclave Herbal Powder Sterilization Machine for Plant
- Portable Digital Display Automatic Laboratory Sterilizer Lab Autoclave for Sterilization Pressure
- Portable High Pressure Laboratory Autoclave Steam Sterilizer for Lab Use
People Also Ask
- Why are hydrothermal autoclaves lined with PTFE selected for MCC-1 static leaching? Ensure Chemical Integrity
- How is an autoclave used to sterilize various requirement in the laboratory? A Guide to Effective Steam Sterilization
- Is autoclave suitable for all materials? The Definitive Guide to Safe Sterilization
- Should glassware be autoclaved? A Guide to Safe and Effective Sterilization
- Why Use a PTFE-Lined Stainless Steel Autoclave for CeO2 Nanosheets? Essential Equipment for Purity & Control
- How does temperature affect sterilization? Unlock the Science of Heat-Based Microbial Destruction
- Why is a PTFE-lined autoclave necessary for Na-Ce-modified-SBA-15 catalyst aging? Ensuring Structural Integrity
- What autoclave is used for sterilization? The Definitive Guide to Steam Sterilization



















