At its core, a chemical reactor is far more than a simple vessel for mixing chemicals. It is a highly engineered system designed to create the optimal environment for a chemical reaction to occur efficiently, safely, and economically. Its advantages stem from its ability to precisely control the conditions that govern the transformation of raw materials into valuable products.
The fundamental advantage of a chemical reactor is its power to translate a chemical equation into a controlled, scalable, and predictable industrial process. It provides the command center for manipulating thermodynamics and kinetics to achieve a desired outcome.

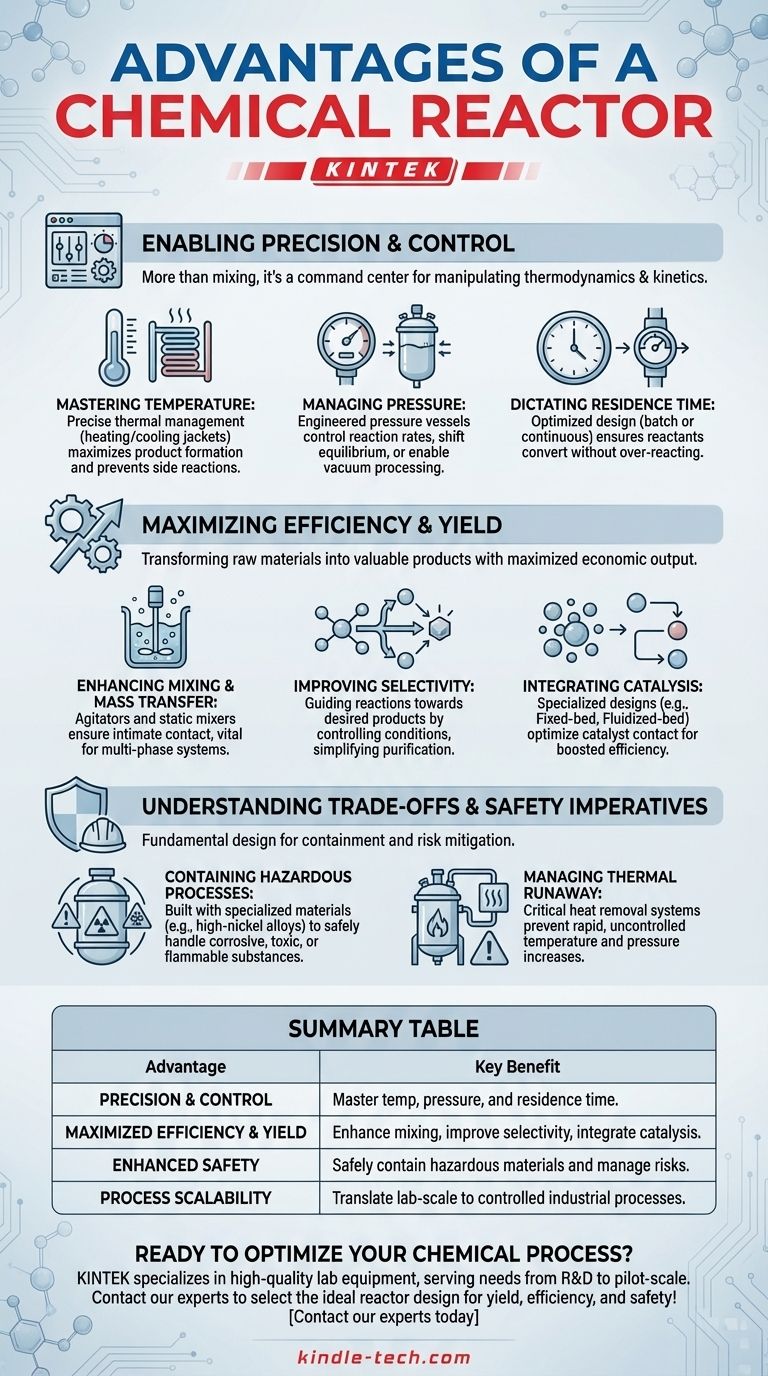
Enabling Precision and Control
The primary function of a reactor is to impose control over a naturally chaotic chemical transformation. This control is the difference between a laboratory curiosity and a viable manufacturing process.
Mastering Temperature
A reactor provides mechanisms for precise thermal management. Through features like heating/cooling jackets, internal coils, or external heat exchangers, operators can maintain a specific temperature profile.
This control is critical for maximizing the formation of the desired product while minimizing energy-wasteful or hazardous side reactions. Some reactions must be kept at a constant temperature (isothermal), while others are allowed to proceed without heat exchange (adiabatic).
Managing Pressure
Many reactions are highly sensitive to pressure. A reactor is a pressure vessel, engineered to operate safely under high pressure or vacuum.
Increasing pressure can increase reaction rates and shift the chemical equilibrium toward the products, leading to higher conversion. Conversely, operating under a vacuum can help remove a product as it forms, also driving the reaction forward.
Dictating Residence Time
The amount of time reactants spend inside the reactor, known as residence time, is a crucial variable. The reactor's design—whether it's a tank for batch processing or a long tube for continuous flow—directly dictates this.
By controlling the flow rate and volume, engineers ensure that the reactants have enough time to convert into products but not so much time that undesired subsequent reactions occur.
Maximizing Efficiency and Yield
In an industrial context, efficiency is profit. A well-designed reactor is a machine for maximizing the economic output of a given chemical process.
Enhancing Mixing and Mass Transfer
Reactions can only happen when molecules collide. A reactor uses agitators, baffles, or static mixers to ensure that reactants are intimately mixed, overcoming physical barriers to reaction known as mass transfer limitations.
This is especially vital in multi-phase systems, such as gas-liquid or liquid-liquid reactions, where contact between the phases is essential for the reaction to proceed.
Improving Selectivity
Most chemical processes can produce a range of products, but only one is typically desired. Selectivity is the measure of how much desired product is made compared to undesired byproducts.
By carefully controlling temperature, pressure, and concentration gradients within the reactor, engineers can guide the reaction down the most favorable pathway. High selectivity dramatically simplifies downstream purification, reducing overall plant cost.
Integrating Catalysis
Catalysts are substances that speed up a reaction without being consumed. Many industrial reactors are designed specifically to optimize the use of a catalyst.
Designs like fixed-bed reactors (where the fluid flows through a static bed of catalyst particles) or fluidized-bed reactors (where the catalyst is suspended in the fluid) maximize the contact between reactants and the catalyst surface, boosting process efficiency.
Understanding the Trade-offs and Safety Imperatives
A reactor's design is not just about efficiency; it is fundamentally about safety. The choice of reactor type involves critical trade-offs between performance and risk mitigation.
Containing Hazardous Processes
Chemical reactors provide containment. They are built from specialized materials like stainless steel, high-nickel alloys, or glass-lined steel to safely handle corrosive, toxic, flammable, or explosive substances. This protects operators, the facility, and the environment.
Managing Thermal Runaway
The most significant risk in many chemical processes is a thermal runaway, where an exothermic (heat-producing) reaction generates heat faster than the reactor can remove it. This leads to a rapid, uncontrolled increase in temperature and pressure, potentially causing an explosion.
A reactor's heat removal system is its most critical safety feature. Its design and capacity must be sufficient to manage the worst-case scenario.
The Conflict Between Ideal Models and Reality
No single reactor is perfect for every application. A Batch Reactor offers great flexibility for producing multiple products but has lower throughput and higher labor costs. A Plug Flow Reactor (PFR) can achieve very high conversion but offers poor temperature control for strongly exothermic reactions. The choice always involves balancing cost, throughput, flexibility, and safety.
Making the Right Choice for Your Process
The optimal reactor is defined by the specific goals of the chemical process. The advantages you seek to maximize will determine the best design.
- If your primary focus is large-scale, continuous production: A Plug Flow Reactor (PFR) or a series of Continuous Stirred-Tank Reactors (CSTRs) is often best for maximizing conversion and throughput.
- If your primary focus is manufacturing flexibility for multiple products: A Batch Reactor provides the versatility to handle different recipes and scales with a lower initial capital investment.
- If your primary focus is managing a highly exothermic or dangerous reaction: A CSTR offers superior temperature control due to its uniform mixing, effectively preventing hot spots and thermal runaway.
- If your primary focus is a gas-solid catalytic reaction: A specialized design like a Fixed-Bed or Fluidized-Bed Reactor is necessary to maximize the interaction between the gas and the catalyst.
Ultimately, a chemical reactor is the tool that empowers engineers to impose order on chemistry, transforming raw potential into tangible value.
Summary Table:
| Advantage | Key Benefit |
|---|---|
| Precision & Control | Master temperature, pressure, and residence time for optimal reaction conditions. |
| Maximized Efficiency & Yield | Enhance mixing, improve selectivity, and integrate catalysis for higher output. |
| Enhanced Safety | Safely contain hazardous materials and manage thermal runaway risks. |
| Process Scalability | Translate lab-scale reactions into predictable, controlled industrial processes. |
Ready to optimize your chemical process with the right reactor? KINTEK specializes in high-quality lab equipment and consumables, serving laboratory needs from R&D to pilot-scale production. Our expertise can help you select the ideal reactor design—whether batch, continuous flow, or catalytic—to maximize your yield, efficiency, and safety. Contact our experts today to discuss your specific application and discover how we can bring precision and reliability to your lab!
Visual Guide
Related Products
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Customizable Laboratory High Temperature High Pressure Reactors for Diverse Scientific Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- Super Sealed Electrolytic Electrochemical Cell
People Also Ask
- Why are high-pressure autoclaves essential for preparing bio-based polyamide curing agents from dimeric acid?
- What is the purpose of using high-purity argon gas in a high-pressure reactor? Ensure Precise Corrosion Test Data
- Why are sealed laboratory reaction vessels necessary in the hydrothermal synthesis of zeolites? Ensure Purity and Yield
- What is the role of high-pressure reactors in the study of alloy oxidation? Essential Tools for Supercritical Research
- What is the contribution of a hydrothermal reactor to graded pore construction? Precision Templates for TAS



















