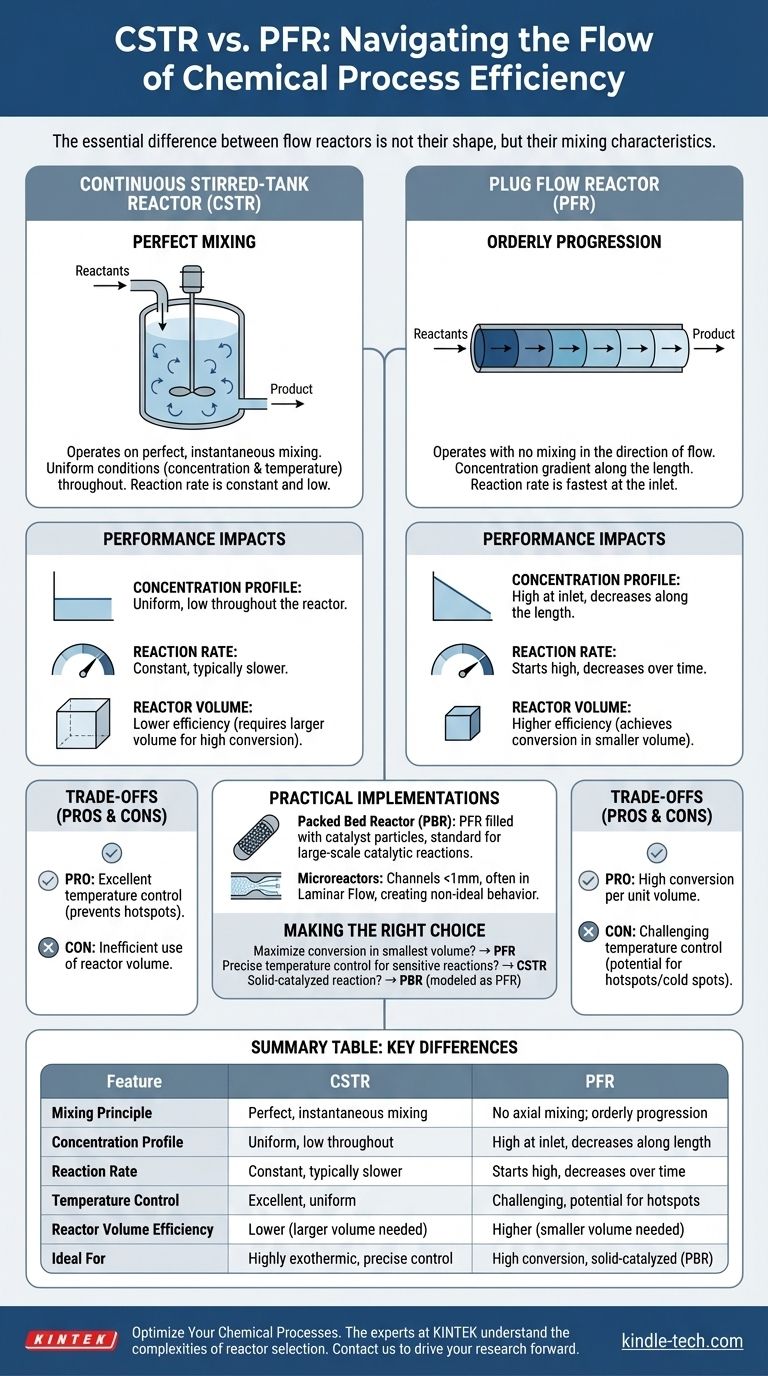
At its core, there are two fundamental ideal types of flow reactors used in chemical engineering: the Continuous Stirred-Tank Reactor (CSTR) and the Plug Flow Reactor (PFR). The CSTR operates on the principle of perfect mixing, resulting in uniform conditions throughout, while the PFR operates with no mixing in the direction of flow, creating a gradient of properties along its length. Nearly all practical flow reactors are designed to approximate one of these two ideal behaviors.
The essential difference between flow reactors is not their shape, but their mixing characteristics. Your choice between a perfectly mixed system (CSTR) and an unmixed, progressing system (PFR) will fundamentally dictate your process's efficiency, control, and final output.

The Two Fundamental Models of Flow
To understand any real-world reactor, you must first grasp the two ideal models they are based on. These models define the boundaries of how reactants can be processed in a continuous system.
The Continuous Stirred-Tank Reactor (CSTR): Perfect Mixing
A CSTR is an idealized model of a reactor where perfect, instantaneous mixing occurs. Imagine a large pot of soup where new ingredients are constantly added, and soup is constantly removed, while a powerful mixer keeps the entire pot perfectly uniform at all times.
The key assumption is that the concentration and temperature of the material exiting the reactor are identical to the conditions at every single point inside the reactor. This means reactions occur at a constant, and typically low, reactant concentration.
The Plug Flow Reactor (PFR): Orderly Progression
A PFR, often visualized as a long tube, models a reactor where fluid flows as a series of discrete "plugs." Each plug is perfectly mixed within itself (radially), but there is absolutely no mixing with the plug in front of it or behind it (axially).
This is like an assembly line. Each plug of reactant enters the reactor and moves along its length, with the reaction progressing over time. Consequently, reactant concentration is high at the inlet and decreases continuously along the length of the reactor.
How Reactor Type Dictates Performance
The difference in mixing directly impacts reaction rates, reactor size, and temperature management, which are the critical performance indicators for a chemical process.
Concentration Profiles and Reaction Rates
For most reactions, the rate is fastest when reactant concentrations are highest.
In a PFR, the reaction begins at a high rate at the inlet where concentration is high and slows down as the reactants are consumed along the tube. It takes full advantage of the initial high concentration.
In a CSTR, the fresh feed immediately mixes with the entire reactor volume, and the concentration instantly drops to the final, low exit concentration. Therefore, the entire reaction takes place at the slowest rate.
Conversion and Reactor Volume
This difference in reaction rate has a massive impact on efficiency. To achieve the same amount of chemical conversion for most standard reactions, a PFR almost always requires a smaller reactor volume than a CSTR.
The CSTR's low, uniform reaction rate means it needs a much larger volume to give the molecules enough time to react to the desired extent. This is one of the most significant practical distinctions between the two types.
Practical Implementations: PBR and Microreactors
In industry, these ideal models are adapted into practical designs.
A Packed Bed Reactor (PBR) is a PFR filled with solid catalyst particles. It is the workhorse for large-scale gas-phase catalytic reactions, like ammonia synthesis or petroleum refining, and its behavior is modeled as a PFR.
Microreactors, with channels smaller than a millimeter, often operate in a Laminar Flow Regime. While still tubular, the lack of turbulence means mixing is not perfect across the channel, creating another type of non-ideal behavior that must be managed.
Understanding the Trade-offs
Choosing a reactor is not about finding the "best" one, but about balancing competing engineering priorities. Neither model is universally superior.
CSTR: Excellent Control vs. Lower Efficiency
The primary advantage of a CSTR is its superb temperature control. The large, well-mixed volume acts as a heat sink, easily absorbing or dissipating the heat of reaction. This makes it ideal for highly exothermic reactions where preventing "hotspots" is a critical safety concern.
Its main drawback is the inefficient use of reactor volume. To achieve very high conversion (e.g., >99%), a CSTR's required volume can become impractically large.
PFR: High Efficiency vs. Thermal Gradients
A PFR's strength is its high conversion per unit volume, making it highly efficient and cost-effective for many processes.
Its weakness is the potential for poor temperature control. Strong exothermic reactions can create dangerous hotspots along the reactor length, while endothermic reactions can create cold spots that quench the reaction. Managing these thermal gradients is a significant engineering challenge.
Series Configurations: The Best of Both Worlds
In practice, engineers often combine reactors. For example, a process might start with a CSTR to handle the bulk of a highly exothermic reaction under tight temperature control, followed by a PFR to efficiently achieve high final conversion.
Making the Right Choice for Your Process
Your decision must be driven by the specific chemistry and operational goals of your project.
- If your primary focus is maximizing conversion in the smallest volume: A PFR is generally the more efficient choice for most positive-order reactions.
- If your primary focus is precise temperature control for a sensitive reaction: A CSTR's uniform temperature profile provides superior stability and safety.
- If your primary focus is a solid-catalyzed reaction: A Packed Bed Reactor (PBR), which is modeled as a PFR, is the standard industry implementation.
- If your primary focus is optimizing selectivity in a complex reaction network: The choice is nuanced; a CSTR's low reactant concentration may favor an intermediate product, while a PFR might be better for others, often requiring detailed modeling.
Ultimately, selecting the right reactor is about aligning the physical flow and mixing characteristics of the hardware with the chemical kinetics of your reaction.
Summary Table:
| Feature | Continuous Stirred-Tank Reactor (CSTR) | Plug Flow Reactor (PFR) |
|---|---|---|
| Mixing Principle | Perfect, instantaneous mixing | No axial mixing; orderly progression |
| Concentration Profile | Uniform, low throughout the reactor | High at inlet, decreases along the length |
| Reaction Rate | Constant, typically slower | Starts high, decreases over time |
| Temperature Control | Excellent, uniform temperature | Challenging, potential for hotspots/cold spots |
| Reactor Volume Efficiency | Lower (requires larger volume for high conversion) | Higher (achieves conversion in smaller volume) |
| Ideal For | Highly exothermic reactions, precise temperature control | High conversion efficiency, solid-catalyzed reactions (PBR) |
Optimize Your Chemical Processes with the Right Reactor Technology
Choosing between a CSTR and a PFR is a critical decision that directly impacts your process efficiency, safety, and final product yield. The experts at KINTEK understand these complexities. We specialize in providing high-quality lab equipment and consumables, including reactor systems, to help you achieve precise control and maximum output for your specific chemical reactions.
Whether you are developing a new process or scaling up production, our team can help you select the ideal reactor configuration. Contact us today to discuss how our solutions can enhance your laboratory's capabilities and drive your research forward.
Contact KINTEK for a consultation
Visual Guide
Related Products
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
People Also Ask
- How does a high-pressure reactor demonstrate its value in accelerated aging? Predict Catalyst Durability Fast
- Why are sealed laboratory reaction vessels necessary in the hydrothermal synthesis of zeolites? Ensure Purity and Yield
- What is the contribution of a hydrothermal reactor to graded pore construction? Precision Templates for TAS
- What role does an autoclave play in simulating PWR conditions? Advanced Material Validation for Nuclear Safety
- What roles do autoclaves play in MFI zeolite synthesis? Master Hydrothermal Crystalline Growth



















